当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solidifying Cathode–Electrolyte Interface for Lithium–Sulfur Batteries
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-06-23 , DOI: 10.1002/aenm.202000791 Wen‐Peng Wang 1, 2 , Juan Zhang 1, 2 , Jia Chou 1, 2 , Ya‐Xia Yin 1, 2 , Ya You 3 , Sen Xin 1, 2 , Yu‐Guo Guo 1, 2
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2020-06-23 , DOI: 10.1002/aenm.202000791 Wen‐Peng Wang 1, 2 , Juan Zhang 1, 2 , Jia Chou 1, 2 , Ya‐Xia Yin 1, 2 , Ya You 3 , Sen Xin 1, 2 , Yu‐Guo Guo 1, 2
Affiliation
|
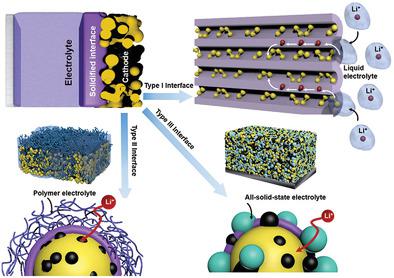
Lithium–sulfur (Li–S) batteries, with their distinct advantages in energy output, cost, and environmental benignancy, have been recognized as one of the most promising candidates for near‐future energy storage markets. However, the energy storage technology based on Li–S systems, even at the single cell level, is far from commercialization. The implementation of the technology is hindered by unstable electrochemistry at the electrode–electrolyte interface, especially the cathode–electrolyte interface. In cases where the cathode builds a solid–liquid interface with the electrolyte, strong interactions between discharge intermediates of S and solvent molecules of the liquid electrolyte lead to continuous loss of active S species from the cathode to the anode through an electrochemical shuttle process, and hampers the cycling performance of the battery. By solidifying the cathode–liquid interface, the polysulfide–solvent interaction is expected to be alleviated and the Li–S electrochemistry improved. In this Progress Report, the strategies to build a solidified cathode–electrolyte interface in liquid, quasi‐solid‐state and all‐solid‐state Li–S systems are summarized, and the fundamentals of charge transfer and chemical evolutions at the interface are discussed. With these discussions, the rational interfacial design of Li–S batteries is elucidated, toward optimal storage performance and operational durability.
中文翻译:

凝固锂-硫电池的阴极-电解质界面
锂硫(Li–S)电池在能量输出,成本和环境友好性方面具有明显的优势,已被公认为是近将来储能市场最有希望的候选者之一。但是,即使在单电池级别,基于Li–S系统的储能技术也远未实现商业化。该技术的实施受到电极-电解质界面(尤其是阴极-电解质界面)不稳定的电化学作用的阻碍。在阴极与电解质建立固液界面的情况下,S的放电中间体与液体电解质的溶剂分子之间的强烈相互作用会导致活性S物质通过电化学穿梭过程从阴极到阳极不断流失,并影响电池的循环性能。通过固化阴极-液体界面,可以减轻多硫化物-溶剂的相互作用,并改善Li-S电化学性能。在此进展报告中,总结了在液态,准固态和全固态Li-S系统中建立固化阴极-电解质界面的策略,并讨论了界面上电荷转移和化学演化的基本原理。通过这些讨论,阐明了锂锂电池的合理界面设计,以实现最佳的存储性能和操作耐久性。总结了在液态,准固态和全固态Li-S系统中建立固化阴极-电解质界面的策略,并讨论了界面处电荷转移和化学演化的基本原理。通过这些讨论,阐明了锂锂电池的合理界面设计,以实现最佳的存储性能和操作耐久性。总结了在液态,准固态和全固态Li-S系统中建立固化阴极-电解质界面的策略,并讨论了界面处电荷转移和化学演化的基本原理。通过这些讨论,阐明了锂锂电池的合理界面设计,以实现最佳的存储性能和操作耐久性。
更新日期:2020-06-23
中文翻译:

凝固锂-硫电池的阴极-电解质界面
锂硫(Li–S)电池在能量输出,成本和环境友好性方面具有明显的优势,已被公认为是近将来储能市场最有希望的候选者之一。但是,即使在单电池级别,基于Li–S系统的储能技术也远未实现商业化。该技术的实施受到电极-电解质界面(尤其是阴极-电解质界面)不稳定的电化学作用的阻碍。在阴极与电解质建立固液界面的情况下,S的放电中间体与液体电解质的溶剂分子之间的强烈相互作用会导致活性S物质通过电化学穿梭过程从阴极到阳极不断流失,并影响电池的循环性能。通过固化阴极-液体界面,可以减轻多硫化物-溶剂的相互作用,并改善Li-S电化学性能。在此进展报告中,总结了在液态,准固态和全固态Li-S系统中建立固化阴极-电解质界面的策略,并讨论了界面上电荷转移和化学演化的基本原理。通过这些讨论,阐明了锂锂电池的合理界面设计,以实现最佳的存储性能和操作耐久性。总结了在液态,准固态和全固态Li-S系统中建立固化阴极-电解质界面的策略,并讨论了界面处电荷转移和化学演化的基本原理。通过这些讨论,阐明了锂锂电池的合理界面设计,以实现最佳的存储性能和操作耐久性。总结了在液态,准固态和全固态Li-S系统中建立固化阴极-电解质界面的策略,并讨论了界面处电荷转移和化学演化的基本原理。通过这些讨论,阐明了锂锂电池的合理界面设计,以实现最佳的存储性能和操作耐久性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号