Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.saa.2020.118642 Mahmoud A Omar 1 , Hytham M Ahmed 2 , Hany A Batakoushy 2 , Mohamed A Abdel Hamid 3
|
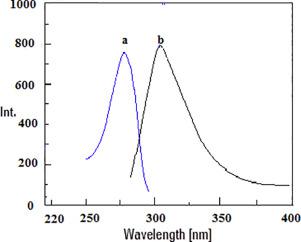
A new, valid method was developed for quantitative spectrofluorimetric analysis of dapagliflozin (DGF) in its pure form and its commercially available tablets (Farxiga®). The proposed analytical method based on measurement of fluorescence intensity for DGF at 303 nm after excitation at 278 nm. Various experimental parameters influencing the fluorescence of DGF were examined to produce the optimal conditions. DGF was successively assayed in concentration range of (100–1000 ng mL−1) with lower detection limit (LOD) of 26.49 and quantitation limit (LOQ) was 79.48 ng mL−1. The suggested method was validated according to ICH guidelines for the estimation of DGF in its pure form and its new dosage form with percent recovery of 100.43 ± 1.15. The proposed method was adapted to examine DGF in content uniformity testing according to United States Pharmacopeia guidelines. This method can be used in routine analysis of DGF in quality control lab.
中文翻译:

荧光光谱法测定纯形式的达格列净及其片剂的含量;适用于内容一致性测试。
开发了一种新的有效方法,用于定量测定其纯净形式的达格列净(DGF)及其市售片剂(Farxiga®)的荧光光谱。基于在278 nm激发后在303 nm下测量DGF荧光强度的分析方法。检查了影响DGF荧光的各种实验参数,以产生最佳条件。DGF的浓度范围为(100–1000 ng mL -1),检测下限(LOD)为26.49,定量下限(LOQ)为79.48 ng mL -1。根据ICH指南验证了所建议的方法,以估算其纯净形式和其新剂型的DGF,回收率为100.43±1.15。根据美国药典指南,建议的方法适用于检查含量均匀性测试中的DGF。该方法可用于质量控制实验室对DGF的常规分析。









































 京公网安备 11010802027423号
京公网安备 11010802027423号