当前位置:
X-MOL 学术
›
Process Saf. Environ. Prot.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recovery of pentoses-containing olive stones for their conversion into furfural in the presence of solid acid catalysts
Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.psep.2020.06.033 I. Fúnez-Núñez , C. García-Sancho , J.A. Cecilia , R. Moreno-Tost , L. Serrano-Cantador , P. Maireles-Torres
Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.psep.2020.06.033 I. Fúnez-Núñez , C. García-Sancho , J.A. Cecilia , R. Moreno-Tost , L. Serrano-Cantador , P. Maireles-Torres
|
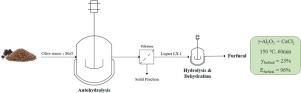
Abstract Olive stones were employed as feedstock for furfural production in two stages: 1) autohydrolysis of hemicellulosic fraction to recover their pentoses, mainly xylose, and 2) subsequent dehydration of pentoses into furfural. Autohydrolysis step was optimized by using different experimental conditions (temperature: 160−200 °C and time: 30−75 min), giving rise to liquors with different xylose concentrations, since hydrolysis was incomplete in some cases. The combined use of a commercial γ-Al2O3 and CaCl2 led to total hydrolysis of non-hydrolyzed pentosans after autohydrolysis step, and the subsequent dehydration of pentoses into furfural. The maximum values of furfural yield and efficiency were 23 and 96 %, respectively, after only 60 min at 150 °C by using liquor obtained by autohydrolysis at 180 °C and 30 min (L5.1) as source of pentoses. This liquor, L5.1, provided better catalytic results than other liquors which had shown higher xylose concentration after autohydrolysis, probably due to these latter also exhibited a higher concentration of organic acids; thus, the presence of organic acids, such as acetic and lactic acids, could promote undesired reactions leading to lower furfural yields. Finally, γ-Al2O3 was more effective for furfural production under these experimental conditions than other solid acid catalysts, such as mesoporous Nb2O5, Nb-doped SBA-15 and Zr-doped HMS silicas, probably due to alumina has a higher density of acid sites.
中文翻译:

在固体酸催化剂存在下回收含戊糖的橄榄核,将其转化为糠醛
摘要 橄榄核被用作糠醛生产的原料,分为两个阶段:1) 半纤维素部分的自水解以回收其戊糖,主要是木糖,以及 2) 戊糖随后脱水成糠醛。通过使用不同的实验条件(温度:160-200°C 和时间:30-75 分钟)优化自水解步骤,产生具有不同木糖浓度的液体,因为在某些情况下水解不完全。商业γ-Al2O3 和CaCl2 的组合使用导致未水解戊聚糖在自水解步骤后完全水解,随后戊糖脱水成糠醛。糠醛产率和效率的最大值分别为 23% 和 96%,在 150 °C 下仅 60 分钟后,使用通过在 180 °C 和 30 分钟 (L5.1) 下自动水解获得的液体作为戊糖来源。这种液体,L5.1,比其他在自水解后表现出更高木糖浓度的液体提供了更好的催化效果,这可能是因为后者也表现出更高浓度的有机酸;因此,有机酸(例如乙酸和乳酸)的存在会促进不希望的反应,从而导致糠醛产率降低。最后,在这些实验条件下,γ-Al2O3 比其他固体酸催化剂(如介孔 Nb2O5、Nb 掺杂的 SBA-15 和 Zr 掺杂的 HMS 二氧化硅)更有效地生产糠醛,这可能是因为氧化铝具有更高的酸性位点密度. 有机酸(例如乙酸和乳酸)的存在会促进不希望的反应,从而导致糠醛产率降低。最后,在这些实验条件下,γ-Al2O3 比其他固体酸催化剂更有效地生产糠醛,例如介孔 Nb2O5、Nb 掺杂的 SBA-15 和 Zr 掺杂的 HMS 二氧化硅,这可能是因为氧化铝具有更高的酸性位点密度. 有机酸(例如乙酸和乳酸)的存在会促进不希望的反应,从而导致糠醛产率降低。最后,在这些实验条件下,γ-Al2O3 比其他固体酸催化剂更有效地生产糠醛,例如介孔 Nb2O5、Nb 掺杂的 SBA-15 和 Zr 掺杂的 HMS 二氧化硅,这可能是因为氧化铝具有更高的酸性位点密度.
更新日期:2020-11-01
中文翻译:

在固体酸催化剂存在下回收含戊糖的橄榄核,将其转化为糠醛
摘要 橄榄核被用作糠醛生产的原料,分为两个阶段:1) 半纤维素部分的自水解以回收其戊糖,主要是木糖,以及 2) 戊糖随后脱水成糠醛。通过使用不同的实验条件(温度:160-200°C 和时间:30-75 分钟)优化自水解步骤,产生具有不同木糖浓度的液体,因为在某些情况下水解不完全。商业γ-Al2O3 和CaCl2 的组合使用导致未水解戊聚糖在自水解步骤后完全水解,随后戊糖脱水成糠醛。糠醛产率和效率的最大值分别为 23% 和 96%,在 150 °C 下仅 60 分钟后,使用通过在 180 °C 和 30 分钟 (L5.1) 下自动水解获得的液体作为戊糖来源。这种液体,L5.1,比其他在自水解后表现出更高木糖浓度的液体提供了更好的催化效果,这可能是因为后者也表现出更高浓度的有机酸;因此,有机酸(例如乙酸和乳酸)的存在会促进不希望的反应,从而导致糠醛产率降低。最后,在这些实验条件下,γ-Al2O3 比其他固体酸催化剂(如介孔 Nb2O5、Nb 掺杂的 SBA-15 和 Zr 掺杂的 HMS 二氧化硅)更有效地生产糠醛,这可能是因为氧化铝具有更高的酸性位点密度. 有机酸(例如乙酸和乳酸)的存在会促进不希望的反应,从而导致糠醛产率降低。最后,在这些实验条件下,γ-Al2O3 比其他固体酸催化剂更有效地生产糠醛,例如介孔 Nb2O5、Nb 掺杂的 SBA-15 和 Zr 掺杂的 HMS 二氧化硅,这可能是因为氧化铝具有更高的酸性位点密度. 有机酸(例如乙酸和乳酸)的存在会促进不希望的反应,从而导致糠醛产率降低。最后,在这些实验条件下,γ-Al2O3 比其他固体酸催化剂更有效地生产糠醛,例如介孔 Nb2O5、Nb 掺杂的 SBA-15 和 Zr 掺杂的 HMS 二氧化硅,这可能是因为氧化铝具有更高的酸性位点密度.











































 京公网安备 11010802027423号
京公网安备 11010802027423号