当前位置:
X-MOL 学术
›
Process Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modifying alcalase activity and stability by immobilization onto chitosan aiming at the production of bioactive peptides by hydrolysis of tilapia skin gelatin
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.procbio.2020.06.019 Paiva dos Santos Kimberle , Mellinger-Silva Carolina , Iraidy Santa Brígida Ana , Rocha Barros Gonçalves Luciana
Process Biochemistry ( IF 3.7 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.procbio.2020.06.019 Paiva dos Santos Kimberle , Mellinger-Silva Carolina , Iraidy Santa Brígida Ana , Rocha Barros Gonçalves Luciana
|
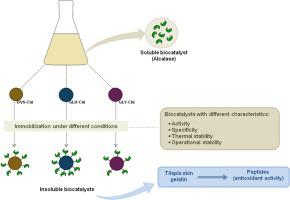
Abstract The protease from Bacillus licheniformis, commercially known as Alcalase®, was insolubilized and stabilized by immobilization onto activated chitosan. Activation with different agents, such as glutaraldehyde (GLU-Chi), glyoxyl (GLY-Chi) and divinyl sulfone (DVS-Chi) was investigated. The effect of the immobilization protocol, for instance different pH and times, were also evaluated. GLU-Chi showed the highest activity (35.6UNPA/g) with the smallest substrate (N-Boc- l -alanine p-nitrophenyl-ester, NPA), while GLY-Chi showed the highest activity (1.5 UAzocasein/g) using the greatest substrate (azocasein). A 24-h immobilization period was enough to stabilize the enzyme using the three supports under almost all conditions. Operational stability in azocasein hydrolysis was assayed and GLU-Chi showed no activity loss during 5 cycles. DVS-Chi retained around 70 % of its initial activity after the fifth cycle, whereas GLY-Chi activity retained only 10 %. Finally, the biocatalysts were used in the hydrolysis of tilapia skin gelatin aiming the production of peptides with antioxidant activity. The protein hydrolysates obtained using GLU-Chi presented the highest antioxidant activity (36.7 μM Trolox Eq). However, the best results of operational stability were obtained using DVS-Chi, which did not lose its initial activity after 3 consecutive cycles of gelatin hydrolysis.
中文翻译:

通过固定化到壳聚糖上来改变碱性蛋白酶的活性和稳定性,旨在通过水解罗非鱼皮明胶生产生物活性肽
摘要 来自地衣芽孢杆菌的蛋白酶,商业上称为 Alcalase®,通过固定在活化的壳聚糖上而变得不溶解和稳定。研究了不同试剂的活化,例如戊二醛 (GLU-Chi)、乙醛 (GLY-Chi) 和二乙烯基砜 (DVS-Chi)。还评估了固定方案的效果,例如不同的 pH 值和时间。GLU-Chi 以最小的底物(N-Boc-l-丙氨酸对硝基苯基酯,NPA)显示出最高的活性(35.6UNPA/g),而 GLY-Chi 显示出最高的活性(1.5 UAzocasein/g)最大底物(偶氮酪蛋白)。24 小时的固定时间足以在几乎所有条件下使用三种支持物稳定酶。测定了偶氮酪蛋白水解的操作稳定性,并且 GLU-Chi 在 5 个循环期间没有显示活性损失。在第五个循环后,DVS-Chi 保留了其初始活性的 70% 左右,而 GLY-Chi 活性仅保留了 10%。最后,将生物催化剂用于罗非鱼皮明胶的水解,旨在生产具有抗氧化活性的肽。使用 GLU-Chi 获得的蛋白质水解物具有最高的抗氧化活性(36.7 μM Trolox Eq)。然而,使用 DVS-Chi 获得了最佳的操作稳定性结果,它在连续 3 个明胶水解循环后没有失去其初始活性。使用 GLU-Chi 获得的蛋白质水解物具有最高的抗氧化活性(36.7 μM Trolox Eq)。然而,使用 DVS-Chi 获得了最佳的操作稳定性结果,它在连续 3 个明胶水解循环后没有失去其初始活性。使用 GLU-Chi 获得的蛋白质水解物具有最高的抗氧化活性(36.7 μM Trolox Eq)。然而,使用 DVS-Chi 获得了最佳的操作稳定性结果,它在连续 3 个明胶水解循环后没有失去其初始活性。
更新日期:2020-10-01
中文翻译:

通过固定化到壳聚糖上来改变碱性蛋白酶的活性和稳定性,旨在通过水解罗非鱼皮明胶生产生物活性肽
摘要 来自地衣芽孢杆菌的蛋白酶,商业上称为 Alcalase®,通过固定在活化的壳聚糖上而变得不溶解和稳定。研究了不同试剂的活化,例如戊二醛 (GLU-Chi)、乙醛 (GLY-Chi) 和二乙烯基砜 (DVS-Chi)。还评估了固定方案的效果,例如不同的 pH 值和时间。GLU-Chi 以最小的底物(N-Boc-l-丙氨酸对硝基苯基酯,NPA)显示出最高的活性(35.6UNPA/g),而 GLY-Chi 显示出最高的活性(1.5 UAzocasein/g)最大底物(偶氮酪蛋白)。24 小时的固定时间足以在几乎所有条件下使用三种支持物稳定酶。测定了偶氮酪蛋白水解的操作稳定性,并且 GLU-Chi 在 5 个循环期间没有显示活性损失。在第五个循环后,DVS-Chi 保留了其初始活性的 70% 左右,而 GLY-Chi 活性仅保留了 10%。最后,将生物催化剂用于罗非鱼皮明胶的水解,旨在生产具有抗氧化活性的肽。使用 GLU-Chi 获得的蛋白质水解物具有最高的抗氧化活性(36.7 μM Trolox Eq)。然而,使用 DVS-Chi 获得了最佳的操作稳定性结果,它在连续 3 个明胶水解循环后没有失去其初始活性。使用 GLU-Chi 获得的蛋白质水解物具有最高的抗氧化活性(36.7 μM Trolox Eq)。然而,使用 DVS-Chi 获得了最佳的操作稳定性结果,它在连续 3 个明胶水解循环后没有失去其初始活性。使用 GLU-Chi 获得的蛋白质水解物具有最高的抗氧化活性(36.7 μM Trolox Eq)。然而,使用 DVS-Chi 获得了最佳的操作稳定性结果,它在连续 3 个明胶水解循环后没有失去其初始活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号