Biomaterials Advances ( IF 5.5 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.msec.2020.111236 Karim Mohsen 1 , Hassan M E Azzazy 2 , Nageh K Allam 3 , Emad B Basalious 4
|
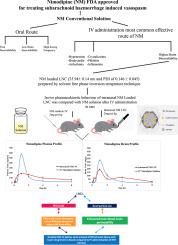
Nimodipine (NM) is FDA-approved drug for treating subarachnoid haemorrhage induced vasospasm. Intravenous (IV) administration, the most common route of NM, causes several side effects such as hypotension, bradycardia, arrhythmias and inflammation at site of administration. The aim of this study was to investigate the capability of intranasal (IN) lipid nanocapsules (LNCs) for effective delivery of NM into the brain. NM LNCs were prepared by solvent free phase inversion temperature technique using D-Optimal mixture design studying the effects of three formulation variables on the properties of the prepared LNCs. The prepared particles were evaluated for particle size, drug payload, PDI, Zeta potential and in-vitro drug release. The optimized NM loaded LNC showed particle size of 35.94 ± 0.14 nm and PDI of 0.146 ± 0.045. The in-vivo pharmacokinetic behaviour of IN NM loaded LNC in blood and brain was compared with NM-solution after IV administration in rats. Results show that IN NM loaded LNC was capable to deliver the same amount of NM at brain tissue with lower drug levels in blood compared with IV administration of the NM solution which is greatly beneficial to minimize the cardiovascular side effects of NM. Contrary to most IN nanocarriers, systemic pathway rather than olfactory pathway plays the major role in brain delivery following IN administration of LNCs. The appropriate brain delivery with lower blood levels and slow elimination propose that intranasal LNCs could provide effective systemic delivery of NM into brain with lower frequency of administration and minimal side effects.
中文翻译:

尼莫地平向脑内全身递送的鼻内脂质纳米胶囊:体外优化和体内药代动力学研究。
尼莫地平(NM)是FDA批准的用于治疗蛛网膜下腔出血引起的血管痉挛的药物。静脉(IV)给药是NM最常见的途径,在给药部位会引起多种副作用,例如低血压,心动过缓,心律不齐和炎症。这项研究的目的是调查鼻内(IN)脂质纳米胶囊(LNCs)将NM有效传递到大脑的能力。通过使用D-最佳混合物设计,研究三种配方变量对制备的LNCs性能的影响,采用无溶剂相转化温度技术制备NM LNC。评价制备的颗粒的粒度,药物有效载荷,PDI,Zeta电位和体外药物释放。优化的NM负载LNC的粒径为35.94±0.14 nm,PDI为0.146±0.045。在大鼠静脉输注后,将IN NM负载的LNC在血液和脑中的体内药代动力学行为与NM溶液进行了比较。结果表明,与静脉内施用NM溶液相比,IN NM加载的LNC能够在脑组织中以较低的血液水平递送相同量的NM,这对于最大程度地降低NM的心血管副作用非常有利。与大多数IN纳米载体相反,在IN施用LNC后,系统途径而非嗅觉途径在脑传递中起主要作用。适当的脑部给药具有较低的血液水平和缓慢的消除作用,提示鼻内LNC可以有效地将NM全身性地递送至脑部,且给药频率较低且副作用最小。











































 京公网安备 11010802027423号
京公网安备 11010802027423号