Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.jmb.2020.06.017 Byungsu Kwon 1 , Taraknath Mandal 2 , Matthew R Elkins 1 , Younghoon Oh 2 , Qiang Cui 3 , Mei Hong 1
|
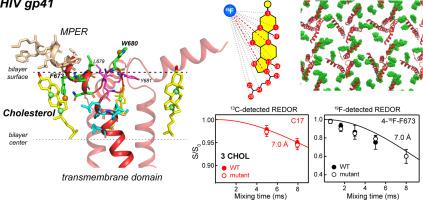
HIV-1 entry into cells is mediated by the fusion protein gp41. Cholesterol plays an important role in this virus–cell fusion, but molecular structural information about cholesterol–gp41 interaction is so far absent. Here, we present experimental and computational data about cholesterol complexation with gp41 in lipid bilayers. We focus on the C-terminal region of the protein, which comprises a membrane-proximal external region (MPER) and the transmembrane domain (TMD). We measured peptide–cholesterol contacts in virus-mimetic lipid bilayers using solid-state NMR spectroscopy, and augmented these experimental data with all-atom molecular dynamics simulations. 2D 19F NMR spectra show correlation peaks between MPER residues and the cholesterol isooctyl tail, indicating that cholesterol is in molecular contact with the MPER–TMD trimer. 19F–13C distance measurements between the peptide and 13C-labeled cholesterol show that C17 on the D ring and C9 at the intersection of B and C rings are ~ 7.0 Å from the F673 side-chain 4-19F. At high peptide concentrations in the membrane, the 19F–13C distance data indicate three cholesterol molecules bound near F673 in each trimer. Mutation of a cholesterol recognition amino acid consensus motif did not change these distances, indicating that cholesterol binding does not require this sequence motif. Molecular dynamics simulations further identify two hotspots for cholesterol interactions. Taken together, these experimental data and simulations indicate that the helix-turn-helix conformation of the MPER–TMD is responsible for sequestering cholesterol. We propose that this gp41–cholesterol interaction mediates virus–cell fusion by recruiting gp41 to the boundary of the liquid-disordered and liquid-ordered phases to incur membrane curvature.
中文翻译:

通过固态核磁共振波谱和分子动力学模拟研究胆固醇与脂质双层中三聚体 HIV 融合蛋白 gp41 的相互作用。
HIV-1 进入细胞是由融合蛋白 gp41 介导的。胆固醇在这种病毒-细胞融合中发挥着重要作用,但迄今为止还缺乏有关胆固醇-gp41 相互作用的分子结构信息。在这里,我们展示了有关脂质双层中胆固醇与 gp41 络合的实验和计算数据。我们重点关注蛋白质的 C 末端区域,其中包括近膜外部区域 (MPER) 和跨膜结构域 (TMD)。我们使用固态核磁共振波谱测量了病毒模拟脂质双层中的肽-胆固醇接触,并通过全原子分子动力学模拟增强了这些实验数据。 2D 19 F NMR 谱显示 MPER 残基和胆固醇异辛基尾部之间的相关峰,表明胆固醇与 MPER-TMD 三聚体存在分子接触。肽与13 C 标记胆固醇之间的19 F– 13 C 距离测量表明,D 环上的 C17 以及 B 环和 C 环交叉处的 C9 距 F673 侧链 4- 19 F 约 7.0 Å。膜中的肽浓度, 19 F- 13 C 距离数据表明每个三聚体中 F673 附近结合了三个胆固醇分子。胆固醇识别氨基酸共有基序的突变不会改变这些距离,表明胆固醇结合不需要该序列基序。分子动力学模拟进一步确定了胆固醇相互作用的两个热点。总而言之,这些实验数据和模拟表明 MPER-TMD 的螺旋-转角-螺旋构象负责隔离胆固醇。 我们提出,这种 gp41-胆固醇相互作用通过将 gp41 招募到液体无序相和液体有序相的边界以引起膜弯曲来介导病毒-细胞融合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号