Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.jfluchem.2020.109595 Yunju Zhang , Yizhen Tang
|
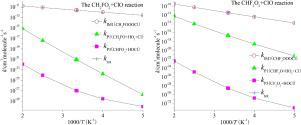
The kinetics and mechanisms of the CH2FO2/CHF2O2 with ClO reactions have been investigated firstly by using the quantum chemical method. The single and triplet potential energy surfaces (PESs) of these two reactions have been obtained by using the BMC-CCSD method based on the geometries obtained at the B3LYP/6-311++G(d,p) level. For the CH2FO2 with ClO reaction, three activated intermediates, IM1 (CH2FOOOCl), IM2 (CH2FOOClO), and IM3 (CH2FOClO2) are generated along the reaction path, and then dissociate to various products. The similar production pathways are investigated for the CHF2O2 with ClO reaction. It should be noted that the isomerization of IM3 (CH2FOClO2) and the 1,3-Cl shift from IM1 (CH2FOOOCl) pathways were not found for the CH2FO2 with ClO reaction. The rate constants for the dominant product pathways are predicted by RRKM-TST theories. The major production pathway is the generation of IM1 (CH2FOOOCl)/IM1 (CHF2OOOCl) at 200-1200 K, and the generation of P1 (CHFO + HO2 +Cl)/P1 (CF2O + HO2 + Cl) by H-migration/elimination become dominant at higher temperatures for the CH2FO2/CHF2O2 + ClO reactions, respectively. The atmospheric lifetimes of CH2FO2 and CHF2O2 in ClO are around 11.38 and 8.65 h, respectively. Furthermore, on the basis of the analysis of the kinetics of all channels through which the addition and abstraction reactions proceed, we expect that the competitive power of reaction channels may vary with experimental conditions for the title reaction. The present study may be helpful for probing the mechanisms of the title reaction and understanding the halogen chemistry.
中文翻译:

CH 2 FO 2 / CHF 2 O 2与ClO反应在大气中的机理和途径的理论研究
首先利用量子化学方法研究了CH 2 FO 2 / CHF 2 O 2的ClO反应动力学和机理。通过使用BMC-CCSD方法基于在B3LYP / 6-311 ++ G(d,p)水平获得的几何结构,已获得了这两个反应的单势和三重势能面(PES)。对于具有ClO反应的CH 2 FO 2,需要使用三种活化的中间体IM1(CH 2 FOOOCl),IM2(CH 2 FOOClO)和IM3(CH 2 FOClO 2)沿着反应路径生成,然后分解为各种产物。研究了具有ClO反应的CHF 2 O 2的相似生产途径。应当注意的是,对于具有ClO反应的CH 2 FO 2,未发现IM3(CH 2 FOClO 2)的异构化和从IM1(CH 2 FOOOCl)途径的1,3-Cl转移。主导产品途径的速率常数由RRKM-TST理论预测。主要的生产途径是在200-1200 K下生成IM1(CH 2 FOOOCl)/ IM1(CHF 2 OOOCl),以及生成P1(CHFO + HO 2 + Cl)/ P1(CF 2在较高的温度下,对于CH 2 FO 2 / CHF 2 O 2 + ClO反应,分别通过H迁移/消除作用生成O + HO 2 + Cl)。在ClO中,CH 2 FO 2和CHF 2 O 2的大气寿命分别约为11.38和8.65 h。此外,基于对所有通道进行加成反应和抽象反应的动力学分析,我们期望反应通道的竞争能力可能随标题反应的实验条件而变化。本研究可能有助于探索标题反应的机理和理解卤素化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号