iScience ( IF 5.8 ) Pub Date : 2020-06-23 , DOI: 10.1016/j.isci.2020.101307 Haiwen Wang 1 , Liyun Liang 1 , Zhirong Guo 1 , Hui Peng 1 , Shuang Qiao 1 , Nemai Saha 2 , Daqian Zhu 1 , Wenbin Zeng 3 , Yunyun Chen 4 , Peng Huang 1 , Shijun Wen 1
|
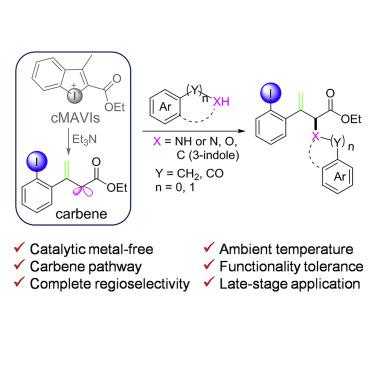
Cross-coupling reactions between aryl iodide and nucleophiles have been well developed. Iodoniums equipped with a reactive C-I(III) bond accelerate cross-coupling reactions of aryl iodide. Among them, cyclic diaryliodoniums are more atom economical; however; they are often in the trap of metal reliance and encounter regioselectivity issues. Now, we have developed a series of highly reactive cyclic monoaryl-vinyl iodoniums that can be tuned to construct C-N, C-O, and C-C bonds without metal catalysis. Under promotion of triethylamine, coupling reactions with aniline, phenol, aromatic acid, and indole proceed rapidly and regioselectively at room temperature. The carbene species is conceptualized as a key intermediate in our mechanism model. Furthermore, the coupling products enable diversity-oriented synthesis strategy to further build up a chemical library of diverse heterocyclic fragments that are in demand in the drug discovery field. Our current work provides a deep insight into the synthetic application of these highly reactive cyclic iodoniums.
中文翻译:

在无金属条件下,将高反应性环状单芳基碘鎓与碳亲核剂一起作为碳发生物进行调节。
芳基碘化物和亲核试剂之间的交叉偶联反应已经得到很好的发展。配有反应性CI(III)键的碘鎓可加速芳基碘化物的交叉偶联反应。其中,环状二芳基碘鎓更原子经济。然而; 它们经常陷入金属依赖的陷阱,并遇到区域选择性问题。现在,我们开发了一系列高反应性的环状单芳基-乙烯基碘鎓,可以调节它们在没有金属催化的情况下构建CN,CO和CC键。在三乙胺的促进下,在室温下,与苯胺,苯酚,芳族酸和吲哚的偶联反应快速且区域选择性地进行。卡宾物质被概念化为我们机理模型中的关键中间体。此外,偶联产品使面向多样性的合成策略能够进一步建立药物发现领域所需的多种杂环片段的化学文库。我们当前的工作为这些高反应性环状碘鎓的合成应用提供了深刻的见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号