Immunology Letters ( IF 4.4 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.imlet.2020.06.011 Angela Santoni 1 , Edoardo Arcuri 2
|
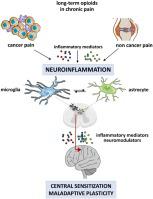
Herein, we summarize the steps of a common scientific path taken by the two Guest Editors, an Anesthesiologist (EA) and an Immunologist (AS), and started 25 years ago at the National Cancer Institute in Rome. When in 1980 WHO codified the usage of opioids for cancer pain relief, it was matter of debate whether only disease progression rather than opioid tolerance were the driving force of opioid escalation. The selective intratumoral accumulation of morphine observed in an experimental xenograft model – the initial scenario of our scientific collaboration – revealed a surprising interaction between the opioid and the opioid receptors expressed by cells of tumor microenvironment. This link could explain the peculiar opioid tolerance and likely hyperalgesia that were observed in the emerging clinical experience of cancer paradoxical pain and suggestive of opioid ambiguity. More elegant cancer pain experimental models, in particular of bone cancer, demonstrated the relevance of inflammatory mediators produced and released by tumor microenvironment cells. These factors were the words of an immune-mediated cross-talk between the tumor and the peripheral and central nervous systems leading to neuroinflammation and consequent pain hypersensitivity, chronicization of acute pain and maladaptive neuroplasticity. Immunology identified in the microglia activation a crucial hub of neuroinflammation and pain centralization. Subsequently the discovery of TLR-4 capacity to bind to opioids on glial cells revealed that they shared the same neuroinflammatory mechanisms underlying cancer and non cancer pain, and could also worsen pain for which they were used.
The late awareness of this knowledge and the poor integration between immunological and pain sciences contributed to the recent severe opioid crisis in the USA (opioid epidemic) with a consequent limitation of long-term use of these drugs in non cancer pain, and generated a new wave of opiophobia. Immunological evidence-based pain therapies are currently quite sophisticated, but only little clinically exploited yet. To save the analgesic use of opioids would require the overcome of their intrinsic ability to cause both analgesia and hyperalgesia in a very ambiguous manner. At moment not to hijack and not to usurp the immune system appears still a very far goal.
中文翻译:

免疫学揭示的阿片类药物的歧义正在改变癌症和非癌性疼痛的知识和治疗方法:叙述性评论。
在此,我们总结了两位客座编辑(麻醉学家(EA)和免疫学家(AS))采取的共同科学路径的步骤,这些步骤是25年前在罗马国家癌症研究所开始的。当世界卫生组织在1980年编纂了使用阿片类药物缓解癌症疼痛的法典时,是否仅因疾病进展而不是阿片类药物耐受性是阿片类药物逐步升级的驱动因素,这是有争议的问题。在实验性异种移植模型中观察到的吗啡选择性肿瘤内蓄积(我们科学合作的初始场景)揭示了阿片样物质与肿瘤微环境细胞表达的阿片样物质受体之间令人惊讶的相互作用。该链接可以解释在癌症悖论性疼痛的新兴临床经验中观察到的独特的阿片类药物耐受性和可能的痛觉过敏,并暗示阿片类药物的歧义性。更优雅的癌症疼痛实验模型,尤其是骨癌,证明了肿瘤微环境细胞产生和释放的炎症介质的相关性。这些因素是肿瘤与周围和中枢神经系统之间免疫介导的串扰的说法,导致神经发炎和随之而来的疼痛超敏反应,急性疼痛的慢性化和适应不良的神经可塑性。在小胶质细胞激活中确定的免疫学是神经炎症和疼痛集中的关键枢纽。
对这种知识的较晚认识以及免疫学和疼痛科学之间的不良融合导致了最近在美国发生的严重阿片类药物危机(阿片类药物的流行),从而限制了这些药物在非癌症性疼痛中长期使用,并产生了新的恐惧症的浪潮。目前,基于免疫学证据的疼痛疗法相当复杂,但在临床上却很少被利用。为了节省镇痛药的使用,需要克服阿片类药物以非常模棱两可的方式引起镇痛和痛觉过敏的内在能力。现在,不劫持和篡夺免疫系统似乎仍然是一个遥远的目标。



























 京公网安备 11010802027423号
京公网安备 11010802027423号