Catalysis Communications ( IF 3.4 ) Pub Date : 2020-06-23 , DOI: 10.1016/j.catcom.2020.106093 Kunlin Li , Gui Liu , Chi Wang , Kai Li , Xin Sun , Xin Song , Ping Ning
|
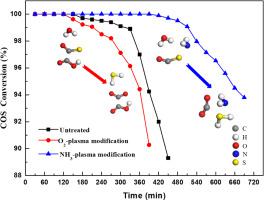
The Fe-based microwave coconut shell activated carbon (Fe/MCSAC) catalyst was surface modified by O2- and NH3-plasma. The catalyst modified by O2-plasma showed a lower catalytic activity than the unmodified sample, but the catalyst modified by NH3-plasma was improved dramatically. Fourier Transform Infrared Spectroscopy (FTIR) results showed that -COOH and -NH2 groups can be generated after by O2- and NH3-plasma modification, respectively. The theoretical calculation results indicated that acidic -COOH group showed some inhibition to the hydrolysis of carbonyl sulfide (COS), but -NH2 could improve it by bonding with the H atoms of H2O.
中文翻译:

在等离子体表面改性过程中引入活性炭表面的酸性和碱性基团可改变COS催化水解活性
Fe基微波椰壳活性炭(Fe / MCSAC)催化剂经O 2-和NH 3-等离子体表面改性。O 2-等离子体改性的催化剂显示出比未改性样品更低的催化活性,但是NH 3-等离子体改性的催化剂得到了显着改善。傅立叶变换红外光谱(FTIR)结果表明,分别通过O 2-和NH 3-等离子体改性可以生成-COOH和-NH 2基团。理论计算结果表明,酸性-COOH基团对羰基硫(COS)的水解有一定的抑制作用,而-NH 2通过与H 2 O的H原子键合可以改善它。











































 京公网安备 11010802027423号
京公网安备 11010802027423号