Brain Research Bulletin ( IF 3.5 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.brainresbull.2020.06.010 S V Demyanenko 1 , V A Dzreyan 1 , A B Uzdensky 1
|
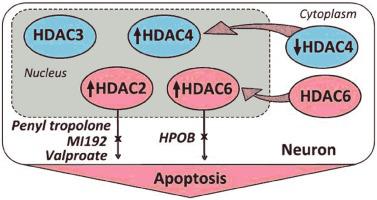
Epigenetic processes play important roles in brain responses to ischemic injury. We studied effects of photothrombotic stroke (PTS, a model of ischemic stroke) on the intracellular level and cellular localization of histone deacetylases HDAC3, HDAC4 and HDAC6 in the rat brain cortex, and tested the potential neuroprotector ability of their inhibitors. The background level of HDAC3, HDAC4 and HDAC6 in the rat cerebral cortex was relatively low. HDAC3 localized in the nuclei of some neurons and few astrocytes. HDAC4 was found in the neuronal cytoplasm. After PTS, their levels in penumbra did not change, but HDAC4 appeared in the nuclei of some cells. Its level in the cytoplasmic, but not nuclear fraction of penumbra decreased at 24, but not 4 h after PTS. HDAC6 was upregulated in neurons and astrocytes in the PTS-induced penumbra, especially in the nuclear fraction. Unlike HDAC3 and HDAC4, HDAC6 co-localized with TUNEL-positive apoptotic cells. Inhibitory analysis confirmed the involvement of HDAC6, but not HDAC3 and HDAC4 in neurodegeneration. HDAC6 inhibitor HPOB, HDAC2/8 inhibitor α-phenyl tropolone, and non-specific histone deacetylase inhibitor sodium valproate, but not HDAC3 inhibitor BRD3308, or HDAC4 inhibitor LMK235, decreased PTS-induced infarction volume in the mouse brain, reduced apoptosis, and recovered the motor behavior. HPOB also restored PTS-impaired acetylation of α-tubulin. α-phenyl tropolone restored acetylation of histone H4 in penumbra cells. These results suggest that histone deacetylases HDAC6 and HDAC2 are the possible molecular targets for anti-ischemic therapy, and their inhibitors α-phenyl tropolone, HBOP and sodium valproate can be considered as promising neuroprotectors.
中文翻译:

大鼠大脑皮层光血栓形成后半暗带中 HDAC6 过表达,而不是 HDAC3 和 HDAC4,以及 α-苯基托酚酮、HPOB 和丙戊酸钠的神经保护作用。
表观遗传过程在脑对缺血性损伤的反应中起重要作用。我们研究了光血栓性中风(PTS,缺血性中风模型)对大鼠大脑皮层中组蛋白去乙酰化酶 HDAC3、HDAC4 和 HDAC6 的细胞内水平和细胞定位的影响,并测试了其抑制剂的潜在神经保护能力。大鼠大脑皮层中HDAC3、HDAC4和HDAC6的背景水平相对较低。HDAC3 位于一些神经元和少数星形胶质细胞的细胞核中。在神经元细胞质中发现了 HDAC4。PTS后,它们在半影区的水平没有变化,但HDAC4出现在一些细胞的细胞核中。在 PTS 后 24 小时而非 4 小时,其在细胞质而非核部分中的水平下降。HDAC6 在 PTS 诱导的半暗带中的神经元和星形胶质细胞中上调,特别是在核部分。与 HDAC3 和 HDAC4 不同,HDAC6 与 TUNEL 阳性凋亡细胞共定位。抑制分析证实了 HDAC6 参与神经变性,而不是 HDAC3 和 HDAC4。HDAC6 抑制剂 HPOB、HDAC2/8 抑制剂 α-苯基托酚酮和非特异性组蛋白去乙酰化酶抑制剂丙戊酸钠,但不是 HDAC3 抑制剂 BRD3308 或 HDAC4 抑制剂 LMK235,可减少 PTS 诱导的小鼠脑梗死体积,减少细胞凋亡,并恢复运动行为。HPOB 还恢复了 PTS 受损的 α-微管蛋白乙酰化。α-苯基托酚酮恢复半影细胞中组蛋白 H4 的乙酰化。这些结果表明组蛋白去乙酰化酶 HDAC6 和 HDAC2 是抗缺血治疗可能的分子靶点,它们的抑制剂 α-苯基托酚酮、











































 京公网安备 11010802027423号
京公网安备 11010802027423号