Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-06-24 , DOI: 10.1016/j.apsb.2020.06.003 Xiaosa Chang , Dejuan Sun , Danfeng Shi , Guan Wang , Yanmei Chen , Kai Zhang , Huidan Tan , Jie Liu , Bo Liu , Liang Ouyang
|
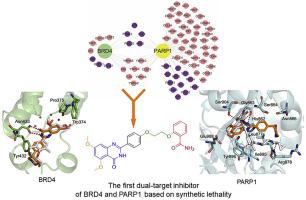
This study was aimed to design the first dual-target small-molecule inhibitor co-targeting poly (ADP-ribose) polymerase-1 (PARP1) and bromodomain containing protein 4 (BRD4), which had important cross relation in the global network of breast cancer, reflecting the synthetic lethal effect. A series of new BRD4 and PARP1 dual-target inhibitors were discovered and synthesized by fragment-based combinatorial screening and activity assays that together led to the chemical optimization. Among these compounds, 19d was selected and exhibited micromole enzymatic potencies against BRD4 and PARP1, respectively. Compound 19d was further shown to efficiently modulate the expression of BRD4 and PARP1. Subsequently, compound 19d was found to induce breast cancer cell apoptosis and stimulate cell cycle arrest at G1 phase. Following pharmacokinetic studies, compound 19d showed its antitumor activity in breast cancer susceptibility gene 1/2 (BRCA1/2) wild-type MDA-MB-468 and MCF-7 xenograft models without apparent toxicity and loss of body weight. These results together demonstrated that a highly potent dual-targeted inhibitor was successfully synthesized and indicated that co-targeting of BRD4 and PARP1 based on the concept of synthetic lethality would be a promising therapeutic strategy for breast cancer.
中文翻译:

共靶向聚(ADP-核糖)聚合酶-1和含溴结构域的蛋白4的喹唑啉-4(3 H)-一衍生物的设计,合成和生物学评估,用于乳腺癌治疗
这项研究旨在设计第一个双靶标小分子抑制剂,共同靶向聚(ADP-核糖)聚合酶-1(PARP1)和含溴结构域的蛋白4(BRD4),它们在乳腺癌的全球网络中具有重要的交叉关系癌症,反映了合成的致死作用。通过基于片段的组合筛选和活性分析发现并合成了一系列新的BRD4和PARP1双靶抑制剂,它们共同导致了化学优化。在这些化合物中,选择了19d并分别表现出针对BRD4和PARP1的微摩尔酶活性。进一步显示化合物19d可有效调节BRD4和PARP1的表达。随后,化合物19d被发现诱导乳腺癌细胞凋亡并刺激细胞周期停滞在G1期。经过药代动力学研究,化合物19d在乳腺癌易感基因1/2(BRCA1 / 2)野生型MDA-MB-468和MCF-7异种移植模型中显示出抗肿瘤活性,没有明显的毒性和体重减轻。这些结果共同表明,成功合成了一种高效的双重靶向抑制剂,并表明基于合成致死性概念的BRD4和PARP1的共同靶向将是一种有希望的乳腺癌治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号