Cellular and Molecular Gastroenterology and Hepatology ( IF 7.1 ) Pub Date : 2020-06-09 , DOI: 10.1016/j.jcmgh.2020.06.001 Trenton T Ross 1 , Collin Crowley 1 , Kenneth L Kelly 1 , Anthony Rinaldi 1 , David A Beebe 1 , Matthew P Lech 2 , Robert V Martinez 2 , Santos Carvajal-Gonzalez 3 , Magalie Boucher 4 , Dinesh Hirenallur-Shanthappa 5 , Jeffrey Morin 5 , Alan C Opsahl 4 , Sarah R Vargas 4 , Kendra K Bence 1 , Jeffrey A Pfefferkorn 1 , William P Esler 1
|
Background & Aims
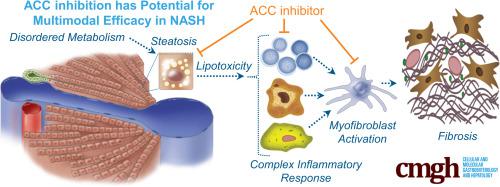
Disordered metabolism, steatosis, hepatic inflammation, and fibrosis contribute to the pathogenesis of nonalcoholic steatohepatitis (NASH). Acetyl-CoA carboxylase (ACC) catalyzes the first committed step in de novo lipogenesis (DNL) and modulates mitochondrial fatty acid oxidation. Increased hepatic DNL flux and reduced fatty acid oxidation are hypothesized to contribute to steatosis. Some proinflammatory cells also show increased dependency on DNL, suggesting that ACC may regulate aspects of the inflammatory response in NASH. PF-05221304 is an orally bioavailable, liver-directed ACC1/2 inhibitor. The present studies sought to evaluate the effects of PF-05221304 on NASH pathogenic factors in experimental model systems.
Methods
The effects of PF-05221304 on lipid metabolism, steatosis, inflammation, and fibrogenesis were investigated in both primary human-derived in vitro systems and in vivo rodent models.
Results
PF-05221304 inhibited DNL, stimulated fatty acid oxidation, and reduced triglyceride accumulation in primary human hepatocytes, and reduced DNL and steatosis in Western diet–fed rats in vivo, showing the potential to reduce hepatic lipid accumulation and potentially lipotoxicity. PF-05221304 blocked polarization of human T cells to proinflammatory but not anti-inflammatory T cells, and suppressed activation of primary human stellate cells to myofibroblasts in vitro, showing direct effects on inflammation and fibrogenesis. Consistent with these observations, PF-05221304 also reduced markers of inflammation and fibrosis in the diethylnitrosamine chemical–induced liver injury model and the choline-deficient, high-fat–fed rat model.
Conclusions
The liver-directed dual ACC1/ACC2 inhibitor directly improved multiple nonalcoholic fatty liver disease/NASH pathogenic factors including steatosis, inflammation, and fibrosis in both human-derived in vitro systems and rat models.
中文翻译:

乙酰辅酶 A 羧化酶抑制可改善模型系统中 NASH 发病机制的多个维度。
背景与目标
代谢紊乱、脂肪变性、肝脏炎症和纤维化是非酒精性脂肪性肝炎 (NASH) 的发病机制。乙酰辅酶A羧化酶 (ACC) 催化从头脂肪生成 (DNL) 的第一个关键步骤并调节线粒体脂肪酸氧化。据推测,肝脏 DNL 通量增加和脂肪酸氧化减少会导致脂肪变性。一些促炎细胞也表现出对 DNL 的依赖性增加,这表明 ACC 可能调节 NASH 中炎症反应的各个方面。 PF-05221304 是一种口服生物可利用的肝脏定向 ACC1/2 抑制剂。本研究旨在评估 PF-05221304 在实验模型系统中对 NASH 致病因素的影响。
方法
在原始人源体外系统和体内啮齿动物模型中研究了 PF-05221304 对脂质代谢、脂肪变性、炎症和纤维形成的影响。
结果
PF-05221304 抑制 DNL,刺激脂肪酸氧化,减少原代人肝细胞中的甘油三酯积累,并减少西方饮食喂养的大鼠体内的 DNL 和脂肪变性,显示出减少肝脏脂质积累和潜在脂毒性的潜力。 PF-05221304 阻断人类 T 细胞向促炎 T 细胞而非抗炎 T 细胞的极化,并在体外抑制原代人星状细胞向肌成纤维细胞的活化,对炎症和纤维形成显示出直接影响。与这些观察结果一致,PF-05221304 还减少了二乙基亚硝胺化学品诱导的肝损伤模型和胆碱缺乏、高脂肪喂养的大鼠模型中的炎症和纤维化标志物。
结论
在人源体外系统和大鼠模型中,针对肝脏的双重 ACC1/ACC2 抑制剂可直接改善多种非酒精性脂肪肝/NASH 致病因素,包括脂肪变性、炎症和纤维化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号