Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aggregation-Enabled Intermolecular Photo[2+2]cycloaddition of Aryl Terminal Olefins by Visible-Light Catalysis
CCS Chemistry ( IF 9.4 ) Pub Date : 2019-11-12 , DOI: 10.31635/ccschem.019.201900049 Zan Liu 1, 2 , Chao Zhou 1, 2 , Tao Lei 1, 2 , Xiao-Lei Nan 1, 2 , Bin Chen 1, 2 , Chen-Ho Tung 1, 2 , Li-Zhu Wu 1, 2
CCS Chemistry ( IF 9.4 ) Pub Date : 2019-11-12 , DOI: 10.31635/ccschem.019.201900049 Zan Liu 1, 2 , Chao Zhou 1, 2 , Tao Lei 1, 2 , Xiao-Lei Nan 1, 2 , Bin Chen 1, 2 , Chen-Ho Tung 1, 2 , Li-Zhu Wu 1, 2
Affiliation
|
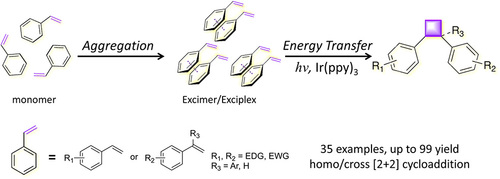
Photo[2+2]cycloaddition of olefins is a sought-after
tool for making cyclobutanes, which are the building blocks for a wide range of biologically active
molecules and natural products. However, the formation of cyclobutane derivatives from aryl terminal olefins is problematic due to their high redox
potential, and high energy in the excited state,
making visible-light-absorbing photocatalyst difficult
to realize either electron or energy transfer to ole-
fins during the photocyclic addition process. Herein,
we report, for the first time, the aggregationinduced photo[2+2]cycloaddition of two olefins by
visible-light catalysis in homogeneous solution. An
array of control experiments and spectroscopic
analyses reveal that the terminal olefins tend to
aggregate to form excimers/exciplexes, which
possess much lower energy than their monomeric
forms, and enables triplet energy transfer from
excited Ir(ppy)3* to supramolecular self-assembly
feasible, leading to homo and cross [2+2] photodimerization of the olefins to fabricate cyclobutanes at ambient conditions in good to excellent
yields.
中文翻译:

可见光催化的芳基末端烯烃的分子间光[2 + 2]环加成反应
烯烃的光[2 + 2]环加成反应是制备环丁烷的抢手工具,而环丁烷是广泛的生物活性分子和天然产物的结构单元。但是,由于芳基末端烯烃的高氧化还原电势和激发态下的高能量,从芳基末端烯烃形成环丁烷衍生物存在问题,这使得吸收可见光的光催化剂难以实现电子或能量转移至烯烃。在光环加成过程中鳍片。在此,我们首次报道了在均相溶液中通过可见光催化的聚集?诱导的两种烯烃的光[2 + 2]环加成反应。一系列对照实验和光谱分析表明,末端烯烃倾向于聚集形成受激准分子/激基复合物,其能量远低于其单体形式,
更新日期:2020-06-24
中文翻译:

可见光催化的芳基末端烯烃的分子间光[2 + 2]环加成反应
烯烃的光[2 + 2]环加成反应是制备环丁烷的抢手工具,而环丁烷是广泛的生物活性分子和天然产物的结构单元。但是,由于芳基末端烯烃的高氧化还原电势和激发态下的高能量,从芳基末端烯烃形成环丁烷衍生物存在问题,这使得吸收可见光的光催化剂难以实现电子或能量转移至烯烃。在光环加成过程中鳍片。在此,我们首次报道了在均相溶液中通过可见光催化的聚集?诱导的两种烯烃的光[2 + 2]环加成反应。一系列对照实验和光谱分析表明,末端烯烃倾向于聚集形成受激准分子/激基复合物,其能量远低于其单体形式,











































 京公网安备 11010802027423号
京公网安备 11010802027423号