Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemical Synthesis of Structurally Defined Phosphorylated Ubiquitins Suggests Impaired Parkin Activation by Phosphorylated Ubiquitins with a Non-Phosphorylated Distal Unit
CCS Chemistry ( IF 9.4 ) Pub Date : 2019-10-04 , DOI: 10.31635/ccschem.019.20190001 Man Pan 1 , Qingyun Zheng 1 , Shuai Gao 1, 2 , Qian Qu 1, 2 , Yuanyuan Yu 1 , Ming Wu 1 , Huan Lan 1 , Yulei Li 3 , Sanling Liu 4 , Jiabin Li 4 , Demeng Sun 4 , Lining Lu 1 , Tian Wang 1 , Wenhao Zhang 1 , Jiawei Wang 1 , Yiming Li 5 , Hong-Gang Hu 3 , Changlin Tian 4 , Lei Liu 1
CCS Chemistry ( IF 9.4 ) Pub Date : 2019-10-04 , DOI: 10.31635/ccschem.019.20190001 Man Pan 1 , Qingyun Zheng 1 , Shuai Gao 1, 2 , Qian Qu 1, 2 , Yuanyuan Yu 1 , Ming Wu 1 , Huan Lan 1 , Yulei Li 3 , Sanling Liu 4 , Jiabin Li 4 , Demeng Sun 4 , Lining Lu 1 , Tian Wang 1 , Wenhao Zhang 1 , Jiawei Wang 1 , Yiming Li 5 , Hong-Gang Hu 3 , Changlin Tian 4 , Lei Liu 1
Affiliation
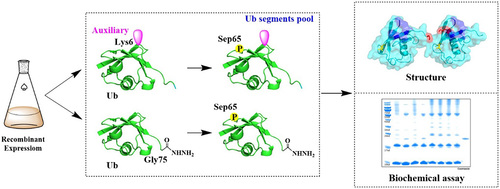
|
Mutations in genes encoding PINK1 (PTEN-induced kinase 1) and Parkin (E3 ubiquitin ligase) are identified in
familial Parkinson’s disease. However, it remains unclear whether the phosphorylated Ub chains activate wildtype Parkin (w-Parkin) or phosphorylated Parkin (p-Parkin), with the consequent expulsion of the damaged
mitochondria. To address this problem, we developed a chemical protein synthesis strategy that integrates
hydrazide-based native chemical ligation, dehydroalanine conjugation, and chemoenzymatic modification to
enable us to access the precise phosphorylated side-chain K6-linked diUbs, namely: (1) UbP
K6Ub, which
carries out phosphorylation at the distal Ub. (2) UbK6UbP
, which carries out phosphorylation at the proximal
Ub. (3) UbP
K6UbP
, which carries out phosphorylation at both distal and proximal Ub units. The structure of
UbP
K6UbP was validated by X-ray crystallographic analysis at 2.2 Å resolution. Using these structurally defined
phosphorylated diUbs to activate Parkin, we found that UbK6UbP could activate p-Parkin but not w-Parkin.
Subsequent biochemical studies showed that UbK6UbP interfered with the transfer of Ub by the Ub–E2 enzyme
conjugate with the thioester-linked intermediate of w-Parkin. Thus our results suggested that w-Parkin and
p-Parkin are activated via two different mechanisms. Collectively, our study exemplifies the utilization of a
novel synthesis strategy for the generation of structurally defined proteins bearing post-translational
modifications and would be useful in elucidating ubiquitin chain signaling and pathways regulation studies
in modern biochemistry and biophysics.
中文翻译:

结构确定的磷酸化泛素的化学合成表明磷酸化泛素与一个非磷酸化的远端单元受损的Parkin活化。
在家族性帕金森氏病中发现了编码PINK1(PTEN诱导的激酶1)和Parkin(E3泛素连接酶)的基因突变。但是,尚不清楚磷酸化的Ub链是否激活野生型帕金(w-Parkin)或磷酸化的帕金(p-Parkin),从而驱逐受损的线粒体。为了解决这个问题,我们开发了一种化学蛋白质合成策略,该策略整合了基于酰肼的天然化学连接,脱氢丙氨酸偶联和化学酶修饰,以使我们能够访问精确的磷酸化侧链K6连接的diUb,即:(1)UbP K6Ub ,在远端Ub进行磷酸化。(2)UbK6UbP,在近端Ub进行磷酸化。(3)UbP K6UbP,在远端和近端Ub单元均进行磷酸化。UbP K6UbP的结构通过X射线晶体学分析以2.2Å分辨率验证。使用这些结构定义的磷酸化diUbs激活Parkin,我们发现UbK6UbP可以激活p-Parkin,而不激活w-Parkin。随后的生化研究表明,UbK6UbP通过与w-Parkin硫酯连接的中间体的Ub-E2酶偶联物干扰了Ub的转移。因此,我们的结果表明,w-Parkin和p-Parkin是通过两种不同的机制激活的。总的来说,我们的研究例证了一种新型合成策略的利用,该策略可用于产生带有翻译后修饰的结构确定的蛋白质,并可用于阐明现代生物化学和生物物理学中的泛素链信号传导和途径调控研究。
更新日期:2020-06-24
中文翻译:

结构确定的磷酸化泛素的化学合成表明磷酸化泛素与一个非磷酸化的远端单元受损的Parkin活化。
在家族性帕金森氏病中发现了编码PINK1(PTEN诱导的激酶1)和Parkin(E3泛素连接酶)的基因突变。但是,尚不清楚磷酸化的Ub链是否激活野生型帕金(w-Parkin)或磷酸化的帕金(p-Parkin),从而驱逐受损的线粒体。为了解决这个问题,我们开发了一种化学蛋白质合成策略,该策略整合了基于酰肼的天然化学连接,脱氢丙氨酸偶联和化学酶修饰,以使我们能够访问精确的磷酸化侧链K6连接的diUb,即:(1)UbP K6Ub ,在远端Ub进行磷酸化。(2)UbK6UbP,在近端Ub进行磷酸化。(3)UbP K6UbP,在远端和近端Ub单元均进行磷酸化。UbP K6UbP的结构通过X射线晶体学分析以2.2Å分辨率验证。使用这些结构定义的磷酸化diUbs激活Parkin,我们发现UbK6UbP可以激活p-Parkin,而不激活w-Parkin。随后的生化研究表明,UbK6UbP通过与w-Parkin硫酯连接的中间体的Ub-E2酶偶联物干扰了Ub的转移。因此,我们的结果表明,w-Parkin和p-Parkin是通过两种不同的机制激活的。总的来说,我们的研究例证了一种新型合成策略的利用,该策略可用于产生带有翻译后修饰的结构确定的蛋白质,并可用于阐明现代生物化学和生物物理学中的泛素链信号传导和途径调控研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号