Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Quaternary Carbon-Centered Benzoindolizidinones via Novel Photoredox-Catalyzed Alkene Aminoarylation: Facile Access to Tylophorine and Analogues
CCS Chemistry ( IF 9.4 ) Pub Date : 2019-09-09 , DOI: 10.31635/ccschem.019.20190018 Chao Zhang 1 , Yi Wang 2 , Yugang Song 1 , Hongying Gao 1 , Yonghui Sun 1 , Xiuyun Sun 1 , Yiqing Yang 1 , Ming He 1 , Zimo Yang 1 , Lingpeng Zhan 3 , Zhi-Xiang Yu 2 , Yu Rao 1
CCS Chemistry ( IF 9.4 ) Pub Date : 2019-09-09 , DOI: 10.31635/ccschem.019.20190018 Chao Zhang 1 , Yi Wang 2 , Yugang Song 1 , Hongying Gao 1 , Yonghui Sun 1 , Xiuyun Sun 1 , Yiqing Yang 1 , Ming He 1 , Zimo Yang 1 , Lingpeng Zhan 3 , Zhi-Xiang Yu 2 , Yu Rao 1
Affiliation
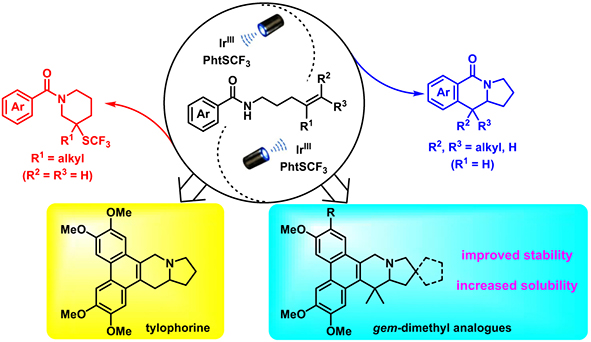
|
Photoredox-catalyzed aminoarylation and thioamination
of unactivated alkenes have been developed,
providing novel synthetic routes to access synthetically
challenging quaternary carbon-centered
benzoindolizidinones and trifluoromethylthiolated
piperidines using readily available starting materials.
Notably, these transformations were enabled by
merging amidyl radical generation from N-alkyl
benzamides with oxidant incorporation. Density
functional theory calculations were performed to
understand the reaction mechanism and to rationalize
the regioselectivities. Moreover, the newly developed
catalytic aminoarylation provided a convenient
synthetic route for natural product tylophorine and
its gem-dimethyl analogues with greatly improved
drug-like properties such as enhanced solubility and
stability.
中文翻译:

通过新型光氧化还原催化的烯烃氨基芳基化反应合成季碳中心的苯并吲哚并二酮类化合物:容易获得酪氨酸和类似物
已经开发了光氧化还原催化的未活化烯烃的氨基芳基化和硫胺化,提供了新颖的合成路线,可使用容易获得的起始原料来获得具有挑战性的季碳中心的苯并吲哚并吲哚酮和三氟甲基硫代哌啶。值得注意的是,这些转化是通过合并由N-烷基苯甲酰胺产生的酰胺基与氧化剂的结合而实现的。进行密度泛函理论计算以了解反应机理并合理化区域选择性。而且,新开发的催化氨基芳基化为天然产物酪氨酸及其宝石-二甲基类似物提供了便利的合成途径,并大大改善了药物样性质,例如增加了溶解度和稳定性。
更新日期:2020-06-24
中文翻译:

通过新型光氧化还原催化的烯烃氨基芳基化反应合成季碳中心的苯并吲哚并二酮类化合物:容易获得酪氨酸和类似物
已经开发了光氧化还原催化的未活化烯烃的氨基芳基化和硫胺化,提供了新颖的合成路线,可使用容易获得的起始原料来获得具有挑战性的季碳中心的苯并吲哚并吲哚酮和三氟甲基硫代哌啶。值得注意的是,这些转化是通过合并由N-烷基苯甲酰胺产生的酰胺基与氧化剂的结合而实现的。进行密度泛函理论计算以了解反应机理并合理化区域选择性。而且,新开发的催化氨基芳基化为天然产物酪氨酸及其宝石-二甲基类似物提供了便利的合成途径,并大大改善了药物样性质,例如增加了溶解度和稳定性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号