Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular α-Oxygenation of Amines via N-Heterocyclic Carbene-Catalyzed Domino Reaction of Aryl Aldehyde: Experiment and DFT Calculation
CCS Chemistry ( IF 9.4 ) Pub Date : 2019-09-09 , DOI: 10.31635/ccschem.019.20190020 Xiang-Yu Chen 1 , Chao-Shen Zhang 2 , Liang Yi 1, 3 , Zhong-Hua Gao 1 , Zhi-Xiang Wang 2 , Song Ye 1, 2
CCS Chemistry ( IF 9.4 ) Pub Date : 2019-09-09 , DOI: 10.31635/ccschem.019.20190020 Xiang-Yu Chen 1 , Chao-Shen Zhang 2 , Liang Yi 1, 3 , Zhong-Hua Gao 1 , Zhi-Xiang Wang 2 , Song Ye 1, 2
Affiliation
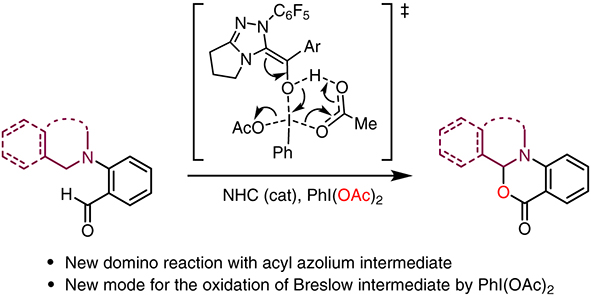
|
We have developed an N-heterocyclic carbene
(NHC)-catalyzed domino reaction with aryl aldehyde
through a simple acyl azolium intermediate
using phenyliodine(III) diacetate (PIDA) as an oxidant.
Controlled experiments and density functional
theory calculations supported a domino two-stage
mechanism from aldehyde/amine to an α-oxygenation
product, including NHC-catalyzed oxidation of
the aldehyde to carboxylic acid via acyl azolium
intermediate, and further, an addition of carboxylate
to iminium intermediate. Our study reveals a
new model for the oxidation of Breslow intermediate
via electrophilic attack of the hydroxyl group by
PIDA.
中文翻译:

N-杂环卡宾催化的芳醛多米诺反应使胺分子内发生α-氧合反应:实验和DFT计算
我们已经开发了一种N-杂环卡宾(NHC)催化的多芳基醛与芳基醛的反应,该反应是通过简单的酰基偶氮中间体,使用二乙酸苯基碘(III)(PIDA)作为氧化剂。受控实验和密度泛函理论计算支持了从醛/胺到α-加氧产物的多米诺两阶段机理,包括NHC催化的经由酰基偶氮中间体将醛氧化为羧酸,以及将羧酸盐添加至亚胺基中间。我们的研究揭示了一种新的模型,用于通过PIDA对羟基进行亲电攻击来氧化Breslow中间体。
更新日期:2020-06-24
中文翻译:

N-杂环卡宾催化的芳醛多米诺反应使胺分子内发生α-氧合反应:实验和DFT计算
我们已经开发了一种N-杂环卡宾(NHC)催化的多芳基醛与芳基醛的反应,该反应是通过简单的酰基偶氮中间体,使用二乙酸苯基碘(III)(PIDA)作为氧化剂。受控实验和密度泛函理论计算支持了从醛/胺到α-加氧产物的多米诺两阶段机理,包括NHC催化的经由酰基偶氮中间体将醛氧化为羧酸,以及将羧酸盐添加至亚胺基中间。我们的研究揭示了一种新的模型,用于通过PIDA对羟基进行亲电攻击来氧化Breslow中间体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号