Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Heterocyclic Carbene-Catalyzed 1,6-Addition of Homoenolate Equivalent Intermediates: Asymmetric Synthesis of Nonspirocyclic Quaternary Oxindoles
CCS Chemistry ( IF 9.4 ) Pub Date : 2019-08-01 , DOI: 10.31635/ccschem.019.20190023 Xiang-Yu Chen 1 , Kun Zhao 1 , Qiang Liu 1 , Ying Zhi 1 , James Ward 2 , Kari Rissanen 2 , Dieter Enders 1
CCS Chemistry ( IF 9.4 ) Pub Date : 2019-08-01 , DOI: 10.31635/ccschem.019.20190023 Xiang-Yu Chen 1 , Kun Zhao 1 , Qiang Liu 1 , Ying Zhi 1 , James Ward 2 , Kari Rissanen 2 , Dieter Enders 1
Affiliation
|
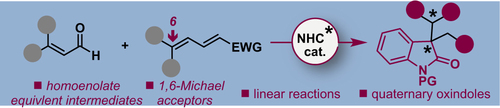
Although there is a growing interest in developing asymmetric 1,6-addition reactions of carbon nucleophiles to Michael acceptors, the corresponding 1,6-addition of homoenolates remains an unsolved problem. Currently, the N-heterocyclic carbene (NHC)-catalyzed cycloadditions of homoenolate equivalent intermediates have achieved widespread success. However, considerable limitations still exist for the linear reactions with electron-deficient alkenes, which are limited to 1,4-Michael acceptors. This report presents the first NHC-catalyzed asymmetric homoenolate addition of enals to 1,6-Michael acceptors. The strategy leads to the challenging nonspirocyclic 3,3-disubstituted oxindoles with two adjacent stereocenters, a quaternary and a trisubstituted one, in good yields and high stereoselectivities with a wide variety of substrates.
中文翻译:

N-杂环卡宾催化的1,6-均烯酸酯等效中间体的加成:非螺环季铵盐的不对称合成
尽管对开发碳亲核试剂与迈克尔受体的不对称1,6-加成反应的兴趣日益浓厚,但相应的均烯酸酯的1,6-加成仍未解决。目前,N-杂环卡宾(NHC)催化的均烯酸酯等效中间体的环加成反应取得了广泛的成功。然而,与缺电子烯烃的线性反应仍然存在相当大的局限性,其限于1,4-迈克尔受体。本报告介绍了NHC催化的1,6-Michael受体烯醛的首次不对称均烯酸酯加成反应。该策略导致具有挑战性的非螺环3,3-二取代的羟吲哚具有两个相邻的立体中心,一个季铵和一个三取代的中心,具有高收率和高立体选择性,并具有多种底物。
更新日期:2020-06-24
中文翻译:

N-杂环卡宾催化的1,6-均烯酸酯等效中间体的加成:非螺环季铵盐的不对称合成
尽管对开发碳亲核试剂与迈克尔受体的不对称1,6-加成反应的兴趣日益浓厚,但相应的均烯酸酯的1,6-加成仍未解决。目前,N-杂环卡宾(NHC)催化的均烯酸酯等效中间体的环加成反应取得了广泛的成功。然而,与缺电子烯烃的线性反应仍然存在相当大的局限性,其限于1,4-迈克尔受体。本报告介绍了NHC催化的1,6-Michael受体烯醛的首次不对称均烯酸酯加成反应。该策略导致具有挑战性的非螺环3,3-二取代的羟吲哚具有两个相邻的立体中心,一个季铵和一个三取代的中心,具有高收率和高立体选择性,并具有多种底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号