Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ni-Catalyzed Enantioconvergent Coupling of Epoxides with Alkenylboronic Acids: Construction of Oxindoles Bearing Quaternary Carbons
CCS Chemistry ( IF 11.2 ) Pub Date : 2019-12-17 , DOI: 10.31635/ccschem.019.201900064 Liang Wu 1 , Guoqiang Yang 1 , Wanbin Zhang 1
CCS Chemistry ( IF 11.2 ) Pub Date : 2019-12-17 , DOI: 10.31635/ccschem.019.201900064 Liang Wu 1 , Guoqiang Yang 1 , Wanbin Zhang 1
Affiliation
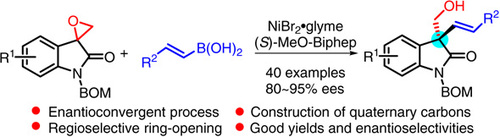
|
We have developed a nickel- nickel/bisphosphine-catalyzed stereoconvergent cross-coupling reaction of epoxides with alkenylboronic acids. Racemic spiroepoxyoxindoles were converted to chiral homoallylic alcohols bearing quaternary carbon stereogenic centers via a stereoablative enantioconvergent transformation. The subsequently fabricated oxindoles-carrying quaternary carbon products were obtained in good yields and enantioselectivity. A wide range of substrates and alkenylboronic acids was tolerated under the catalytic system. This reaction provided a rare example of a nickel-catalyzed enantioselective cross-coupling reaction of tertiary alkyl electrophiles and an enantioconvergent transformation of racemic epoxides, beneficial as a low-cost, sustainable, and efficient catalyst in the preparation of chiral oxindole-containing natural and pharmaceutical compounds.
中文翻译:

镍与烯基硼酸的镍催化对映体偶联:带有季碳原子的吲哚的构建
我们已经开发了镍-镍/双膦催化的环氧化物与烯基硼酸的立体收敛交叉偶联反应。外消旋螺氧基环氧吲哚通过立体消光对映体转化转变为带有季碳立体生成中心的手性均烯丙基醇。以高收率和对映选择性获得了随后制备的带有氧吲哚的季碳产物。在催化体系下可耐受多种底物和烯基硼酸。此反应提供了一个罕见的实例,该反应是叔烷基亲电体的镍催化对映选择性交叉偶联反应和外消旋环氧化物的对映体转化,有利于低成本,可持续,
更新日期:2020-06-24
中文翻译:

镍与烯基硼酸的镍催化对映体偶联:带有季碳原子的吲哚的构建
我们已经开发了镍-镍/双膦催化的环氧化物与烯基硼酸的立体收敛交叉偶联反应。外消旋螺氧基环氧吲哚通过立体消光对映体转化转变为带有季碳立体生成中心的手性均烯丙基醇。以高收率和对映选择性获得了随后制备的带有氧吲哚的季碳产物。在催化体系下可耐受多种底物和烯基硼酸。此反应提供了一个罕见的实例,该反应是叔烷基亲电体的镍催化对映选择性交叉偶联反应和外消旋环氧化物的对映体转化,有利于低成本,可持续,



























 京公网安备 11010802027423号
京公网安备 11010802027423号