Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Borylative Coupling of Vinylazaarenes and Ketones Catalyzed by a Copper(I) Complex
CCS Chemistry ( IF 9.4 ) Pub Date : 2020-03-28 , DOI: 10.31635/ccschem.020.201900102 Xu-Cheng Gan 1 , Liang Yin 1
CCS Chemistry ( IF 9.4 ) Pub Date : 2020-03-28 , DOI: 10.31635/ccschem.020.201900102 Xu-Cheng Gan 1 , Liang Yin 1
Affiliation
|
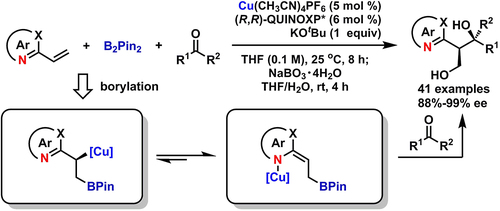
Azaarenes are attractive structural units widely found in chiral pharmaceuticals, agrochemicals, and biologically active natural products. An ongoing strategy has been the construction of new chiral molecules containing azaarenes by virtue of the electron-deficient properties of azaarenes. Herein, an asymmetric three-component coupling of vinylazaarenes, B2Pin2, and ketones was achieved in the presence of a chiral copper(I) catalyst. The reaction favored a broad substrate scope of either vinylazaarenes or ketones. High enantioselectivity was observed in this reaction together with moderate diastereoselectivity, attributable to the relatively small difference of steric repulsion between two proposed transition states. Finally, the synthetic utilities of the product were demonstrated by several beneficial transformation reactions.
中文翻译:

铜(I)配合物催化的乙烯基杂芳烃和酮的不对称硼酸酯偶联
氮杂芳烃是在手性药物,农用化学品和生物活性天然产物中广泛发现的有吸引力的结构单元。正在进行的策略是借助于氮杂芳烃的电子缺陷性质来构建含有氮杂芳烃的新手性分子。在此,在手性铜(I)催化剂的存在下,乙烯基氮杂芳烃,B2Pin2和酮的不对称三组分偶联得以实现。该反应有利于乙烯基氮杂芳烃或酮的广泛的底物范围。在该反应中观察到高的对映选择性以及适度的非对映选择性,这归因于两个提议的过渡态之间的空间排斥相对较小的差异。最后,通过几种有益的转化反应证明了该产品的合成效用。
更新日期:2020-06-24
中文翻译:

铜(I)配合物催化的乙烯基杂芳烃和酮的不对称硼酸酯偶联
氮杂芳烃是在手性药物,农用化学品和生物活性天然产物中广泛发现的有吸引力的结构单元。正在进行的策略是借助于氮杂芳烃的电子缺陷性质来构建含有氮杂芳烃的新手性分子。在此,在手性铜(I)催化剂的存在下,乙烯基氮杂芳烃,B2Pin2和酮的不对称三组分偶联得以实现。该反应有利于乙烯基氮杂芳烃或酮的广泛的底物范围。在该反应中观察到高的对映选择性以及适度的非对映选择性,这归因于两个提议的过渡态之间的空间排斥相对较小的差异。最后,通过几种有益的转化反应证明了该产品的合成效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号