当前位置:
X-MOL 学术
›
Nanoscale Adv.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Boosting activity and selectivity of glycerol oxidation over platinum–palladium–silver electrocatalysts via surface engineering
Nanoscale Advances ( IF 4.7 ) Pub Date : 2020-06-23 , DOI: 10.1039/d0na00252f Yongfang Zhou 1 , Yi Shen 1, 2 , Xuanli Luo 3 , Guo Liu 4 , Yong Cao 4
Nanoscale Advances ( IF 4.7 ) Pub Date : 2020-06-23 , DOI: 10.1039/d0na00252f Yongfang Zhou 1 , Yi Shen 1, 2 , Xuanli Luo 3 , Guo Liu 4 , Yong Cao 4
Affiliation
|
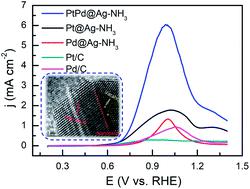
A series of platinum–palladium–silver nanoparticles with tunable structures were synthesized for glycerol electro-oxidation in both alkaline and acidic solutions. Electrochemical results indicate that the catalysts show superior activity in alkaline solutions relative to acidic solutions. In alkaline solutions, the peak current densities of ammonia-etched samples are approximately twice those of saturated-NaCl-etched samples. Ammonia-etched platinum–palladium–silver (PtPd@Ag-NH3) exhibits a peak current density of 9.16 mA cm−2, which is 18.7 and 10 times those of the Pt/C and Pd/C, respectively. The product distribution was analyzed by high performance liquid chromatography. Seven products including oxalic acid, tartronic acid, glyoxylic acid, glyceric acid (GLA), glyceraldehyde (GALD), glycolic acid, and dihydroxyacetone (DHA) were detected. The NH3·H2O etched samples tend to generate more GALD, while the NaCl etched samples have a great potential to produce DHA. The addition of Pd atoms can facilitate glycerol oxidation pathway towards the direction of GALD generation. The Pt@Ag-NaCl possesses the largest DHA selectivity of 79.09% at 1.3 V, while the Pt@Ag-NH3 exhibits the largest GLA selectivity of 45.01% at 0.5 V. The PtPd@Ag-NH3 exhibits the largest C3/C2 ratio of 17.45. The selectivity and product distribution of glycerol electro-oxidation can be tuned by engineering the surface atoms of the as-synthesized catalysts.
中文翻译:

通过表面工程提高铂-钯-银电催化剂上甘油氧化的活性和选择性
合成了一系列结构可调的铂-钯-银纳米粒子,用于在碱性和酸性溶液中进行甘油电氧化。电化学结果表明,催化剂在碱性溶液中的活性优于酸性溶液。在碱性溶液中,氨蚀刻样品的峰值电流密度大约是饱和氯化钠蚀刻样品的两倍。氨蚀刻的铂-钯-银 (PtPd@Ag-NH 3 ) 的峰值电流密度为 9.16 mA cm -2,分别是 Pt/C 和 Pd/C 的 18.7 倍和 10 倍。通过高效液相色谱分析产物分布。检测到草酸、丙醇二酸、乙醛酸、甘油酸(GLA)、甘油醛(GALD)、乙醇酸和二羟基丙酮(DHA)七种产物。NH 3 ·H 2 O 蚀刻的样品倾向于产生更多的GALD,而NaCl 蚀刻的样品具有产生DHA 的巨大潜力。添加 Pd 原子可以促进甘油氧化途径朝着 GALD 生成的方向发展。Pt@Ag-NaCl 在 1.3 V 时具有 79.09% 的最大 DHA 选择性,而 Pt@Ag-NH 3在 0.5 V 时具有 45.01% 的最大 GLA 选择性。PtPd@Ag-NH 3表现出最大的 C3/C2 比率为 17.45。甘油电氧化的选择性和产物分布可以通过设计合成催化剂的表面原子来调节。
更新日期:2020-08-11
中文翻译:

通过表面工程提高铂-钯-银电催化剂上甘油氧化的活性和选择性
合成了一系列结构可调的铂-钯-银纳米粒子,用于在碱性和酸性溶液中进行甘油电氧化。电化学结果表明,催化剂在碱性溶液中的活性优于酸性溶液。在碱性溶液中,氨蚀刻样品的峰值电流密度大约是饱和氯化钠蚀刻样品的两倍。氨蚀刻的铂-钯-银 (PtPd@Ag-NH 3 ) 的峰值电流密度为 9.16 mA cm -2,分别是 Pt/C 和 Pd/C 的 18.7 倍和 10 倍。通过高效液相色谱分析产物分布。检测到草酸、丙醇二酸、乙醛酸、甘油酸(GLA)、甘油醛(GALD)、乙醇酸和二羟基丙酮(DHA)七种产物。NH 3 ·H 2 O 蚀刻的样品倾向于产生更多的GALD,而NaCl 蚀刻的样品具有产生DHA 的巨大潜力。添加 Pd 原子可以促进甘油氧化途径朝着 GALD 生成的方向发展。Pt@Ag-NaCl 在 1.3 V 时具有 79.09% 的最大 DHA 选择性,而 Pt@Ag-NH 3在 0.5 V 时具有 45.01% 的最大 GLA 选择性。PtPd@Ag-NH 3表现出最大的 C3/C2 比率为 17.45。甘油电氧化的选择性和产物分布可以通过设计合成催化剂的表面原子来调节。



























 京公网安备 11010802027423号
京公网安备 11010802027423号