当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ti-catalyzed ring-opening oxidative amination of methylenecyclopropanes with diazenes
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-23 , DOI: 10.1039/d0sc01998d Evan P Beaumier 1 , Amy A Ott 1 , Xuelan Wen 1 , Zachary W Davis-Gilbert 1 , T Alexander Wheeler 1 , Joseph J Topczewski 1 , Jason D Goodpaster 1 , Ian A Tonks 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-06-23 , DOI: 10.1039/d0sc01998d Evan P Beaumier 1 , Amy A Ott 1 , Xuelan Wen 1 , Zachary W Davis-Gilbert 1 , T Alexander Wheeler 1 , Joseph J Topczewski 1 , Jason D Goodpaster 1 , Ian A Tonks 1
Affiliation
|
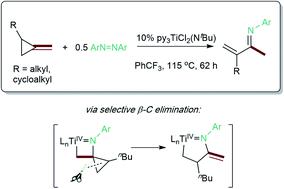
The ring-opening oxidative amination of methylenecyclopropanes (MCPs) with diazenes catalyzed by py3TiCl2(NR) complexes is reported. This reaction selectively generates branched α-methylene imines as opposed to linear α,β-unsaturated imines, which are difficult to access via other methods. Products can be isolated as the imine or hydrolyzed to the corresponding ketone in good yields. Mechanistic investigation via density functional theory suggests that the regioselectivity of these products results from a Curtin–Hammett kinetic scenario, where reversible β-carbon elimination of a spirocyclic [2 + 2] azatitanacyclobutene intermediate is followed by selectivity-determining β-hydrogen elimination of the resulting metallacycle. Further functionalizations of these branched α-methylene imine products are explored, demonstrating their utility as building blocks.
中文翻译:

Ti催化亚甲基环丙烷与二氮烯的开环氧化胺化
报道了 py 3 TiCl 2 (NR) 配合物催化亚甲基环丙烷 (MCP) 与二氮烯的开环氧化胺化反应。该反应选择性地生成支链 α-亚甲基亚胺,而不是通过其他方法难以获得的直链 α,β-不饱和亚胺。产物可以作为亚胺分离或水解成相应的酮,收率良好。通过密度泛函理论进行的机理研究表明,这些产物的区域选择性源自科廷-哈米特动力学场景,其中螺环[2 + 2]氮杂钛环丁烯中间体的可逆β-碳消除,随后是选择性决定的β-氢消除。产生的金属环。探索了这些支链 α-亚甲基亚胺产品的进一步功能化,证明了它们作为构建模块的实用性。
更新日期:2020-07-15
中文翻译:

Ti催化亚甲基环丙烷与二氮烯的开环氧化胺化
报道了 py 3 TiCl 2 (NR) 配合物催化亚甲基环丙烷 (MCP) 与二氮烯的开环氧化胺化反应。该反应选择性地生成支链 α-亚甲基亚胺,而不是通过其他方法难以获得的直链 α,β-不饱和亚胺。产物可以作为亚胺分离或水解成相应的酮,收率良好。通过密度泛函理论进行的机理研究表明,这些产物的区域选择性源自科廷-哈米特动力学场景,其中螺环[2 + 2]氮杂钛环丁烯中间体的可逆β-碳消除,随后是选择性决定的β-氢消除。产生的金属环。探索了这些支链 α-亚甲基亚胺产品的进一步功能化,证明了它们作为构建模块的实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号