当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly enantioselective [3+2] cycloadditions of terminal allenoates with β-trifluoromethyl α,β-enones.
Chemical Communications ( IF 4.3 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0cc02876b Maizhan Li 1 , Wei Zhou
Chemical Communications ( IF 4.3 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0cc02876b Maizhan Li 1 , Wei Zhou
Affiliation
|
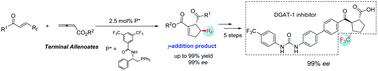
Highly enantio- and diastereoselective phosphine-catalyzed [3+2] cycloadditions of terminal allenoates with β-perfluoroalkyl α,β-enones leading to a range of trifluoromethylated cyclopentenes with two contiguous chiral centers (up to 99% yield with 99% ee) have been developed. Moreover, these reactions could be performed at the gram scale by using only 1 mol% catalyst. The method developed here was also applied to the concise synthesis of trifluoromethylated DGAT-1 inhibitor.
中文翻译:

末端烯丙酸酯与β-三氟甲基α,β-烯酮的高度对映选择性[3 + 2]环加成。
端烯基酸酯与β-全氟烷基α,β-烯酮的高度对映体和非对映体选择性膦催化的[3 + 2]环加成反应产生了一系列具有两个连续手性中心的三氟甲基化环戊烯(产率高达99%,ee达到99%)已开发。而且,这些反应可以仅使用1mol%的催化剂以克为单位进行。此处开发的方法也适用于三氟甲基化DGAT-1抑制剂的简明合成。
更新日期:2020-08-04
中文翻译:

末端烯丙酸酯与β-三氟甲基α,β-烯酮的高度对映选择性[3 + 2]环加成。
端烯基酸酯与β-全氟烷基α,β-烯酮的高度对映体和非对映体选择性膦催化的[3 + 2]环加成反应产生了一系列具有两个连续手性中心的三氟甲基化环戊烯(产率高达99%,ee达到99%)已开发。而且,这些反应可以仅使用1mol%的催化剂以克为单位进行。此处开发的方法也适用于三氟甲基化DGAT-1抑制剂的简明合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号