当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Access to Optically Pure Benzosultams by Superelectrophilic Activation.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.orglett.0c01301 Bastien Michelet 1 , Ugo Castelli 1 , Emeline Appert 1 , Maude Boucher 1 , Kassandra Vitse 1 , Jérôme Marrot 2 , Jérôme Guillard 1 , Agnès Martin-Mingot 1 , Sébastien Thibaudeau 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.orglett.0c01301 Bastien Michelet 1 , Ugo Castelli 1 , Emeline Appert 1 , Maude Boucher 1 , Kassandra Vitse 1 , Jérôme Marrot 2 , Jérôme Guillard 1 , Agnès Martin-Mingot 1 , Sébastien Thibaudeau 1
Affiliation
|
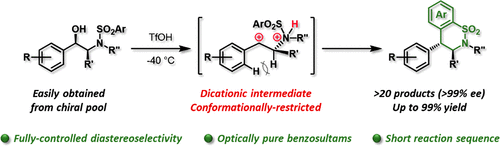
Through superacid activation, N-(arenesulfonyl)-aminoalcohols derived from readily available ephedrines or amino acids undergo an intramolecular Friedel–Crafts reaction to afford enantiopure benzosultams bearing two adjacent stereocenters in high yields with fully controlled diastereoselectivity. Low-temperature NMR spectroscopy demonstrated the crucial role played by the conformationally restricted chiral dicationic intermediates.
中文翻译:

通过超亲电活化获得光学纯净的苯乙磺胺。
通过超强酸活化,源自易于获得的麻黄碱或氨基酸的N-(芳烃磺酰基)-氨基醇会经历分子内Friedel-Crafts反应,以高产率得到对映体纯的苯并阿苏二胺,并具有完全受控的非对映选择性,并带有两个相邻的立体中心。低温NMR光谱表明,构象受限的手性双效中间体具有重要作用。
更新日期:2020-07-02
中文翻译:

通过超亲电活化获得光学纯净的苯乙磺胺。
通过超强酸活化,源自易于获得的麻黄碱或氨基酸的N-(芳烃磺酰基)-氨基醇会经历分子内Friedel-Crafts反应,以高产率得到对映体纯的苯并阿苏二胺,并具有完全受控的非对映选择性,并带有两个相邻的立体中心。低温NMR光谱表明,构象受限的手性双效中间体具有重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号