当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ytterbium-Catalyzed Intramolecular [3 + 2] Cycloaddition based on Furan Dearomatization to Construct Fused Triazoles.
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.orglett.0c01780 Xiaoming Xu 1 , Ying Zhong 1 , Qingzhao Xing 1 , Ziwei Gao 1 , Jing Gou 2 , Binxun Yu 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.orglett.0c01780 Xiaoming Xu 1 , Ying Zhong 1 , Qingzhao Xing 1 , Ziwei Gao 1 , Jing Gou 2 , Binxun Yu 1, 3
Affiliation
|
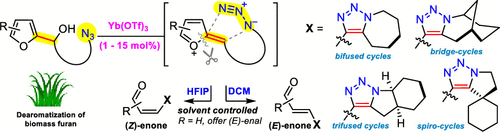
The 1,2,3-triazole-containing polycyclic architecture widely exists in a broad spectrum of synthetic bioactive molecules, and the development of expeditious methods to synthesize these skeletons remains a challenging task. In this work, the catalytic cyclization of biomass-derived 2-furylcarbinols with an azide to form fused triazoles is described. This approach takes advantage of a single catalyst Yb(OTf)3 and operates via a furfuryl-cation-induced intramolecular [3 + 2] cycloaddition/furan ring-opening cascade.
中文翻译:

Fur催化的基于呋喃脱芳香化的分子内[3 + 2]环加成反应,以构建熔融三唑。
含1,2,3-三唑的多环结构广泛存在于广泛的合成生物活性分子中,开发合成这些骨架的快速方法仍然是一项艰巨的任务。在这项工作中,描述了由生物质衍生的2-呋喃基甲醇与叠氮化物的催化环化反应,以形成稠合的三唑。该方法利用单一催化剂Yb(OTf)3的优势,并通过糠基阳离子诱导的分子内[3 + 2]环加成/呋喃开环级联反应进行操作。
更新日期:2020-07-02
中文翻译:

Fur催化的基于呋喃脱芳香化的分子内[3 + 2]环加成反应,以构建熔融三唑。
含1,2,3-三唑的多环结构广泛存在于广泛的合成生物活性分子中,开发合成这些骨架的快速方法仍然是一项艰巨的任务。在这项工作中,描述了由生物质衍生的2-呋喃基甲醇与叠氮化物的催化环化反应,以形成稠合的三唑。该方法利用单一催化剂Yb(OTf)3的优势,并通过糠基阳离子诱导的分子内[3 + 2]环加成/呋喃开环级联反应进行操作。











































 京公网安备 11010802027423号
京公网安备 11010802027423号