当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Covalent vs. Charge-Shift Nature of the Metal−Metal Bond in Transition Metal Complexes: A Unified Understanding
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-23 , DOI: 10.1021/jacs.0c03957 Jyothish Joy 1 , David Danovich 1 , Martin Kaupp 2 , Sason Shaik 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-23 , DOI: 10.1021/jacs.0c03957 Jyothish Joy 1 , David Danovich 1 , Martin Kaupp 2 , Sason Shaik 1
Affiliation
|
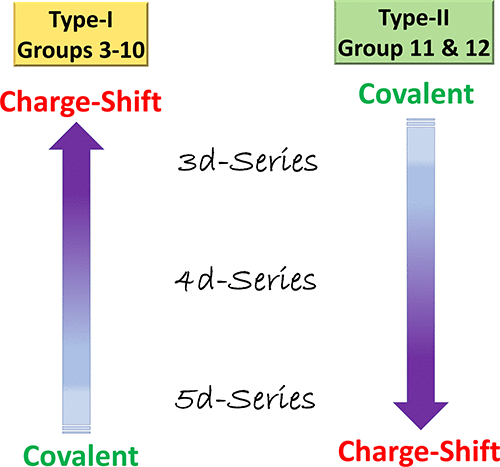
We present here a general conceptualization of the nature of metal-metal (M-M) bonding in transition-metal (TM) complexes across the periods of TM elements, by use of ab-initio valence-bond theory. The calculations reveal a dual-trend: For M-M bonds in groups 7 and 9, the 3d-series forms charge-shift bonds (CSB), while upon moving down to the 5d-series, the bonds become gradually covalent. In contrast, M-M bonds of metals having filled d-orbitals (groups 11 and 12) behave oppositely; initially the M-M bond is covalent, but upon moving down the Periodic Table, the CSB character increases. These trends originate in the radial-distribution-functions of the atomic orbitals, which determine the compactness of the valence-orbitals vis-à-vis the filled semi-core orbitals. Key factors that gauge this compactness are the presence/absence of a radial-node in the valence-orbital and relativistic contraction/expansion of the valence/semi-core orbitals. Whenever these orbital-types are spatially coincident, the covalent bond-pairing is weakened by Pauli-repulsion with the semi-core electrons, and CSB takes over. Thus, for groups 3-10, which possess (n-1)s2(n-1)p6 semi-cores, this spatial-coincidence is maximal at the 3d-transition-metals which consequently form charge-shift M-M bonds. However, in groups 11 and 12, the relativistic effects maximize spatial-coincidence in the third series, wherein the 5d10 core approaches the valence 6s orbital, and the respective Pauli repulsion generates M-M bonds with CSB character. These considerations create a generalized paradigm for M-M bonding in the transition-elements periods, and Pauli repulsion emerges as the factor that unifies CSB over the periods of main-group and transition elements.
中文翻译:

关于过渡金属配合物中金属-金属键的共价与电荷转移性质:统一理解
我们在此通过使用 ab-initio 价键理论,提出了跨 TM 元素周期的过渡金属 (TM) 配合物中金属-金属 (MM) 键合性质的一般概念。计算揭示了双重趋势:对于第 7 组和第 9 组中的 MM 键,3d 系列形成电荷转移键 (CSB),而在向下移动到 5d 系列时,这些键逐渐变成共价键。相比之下,具有填充 d 轨道(第 11 和 12 族)的金属的 MM 键表现相反;最初 MM 键是共价键,但在元素周期表中向下移动时,CSB 特征增加。这些趋势源于原子轨道的径向分布函数,它决定了价轨道相对于填充的半核轨道的紧凑性。衡量这种紧凑性的关键因素是价轨道中径向节点的存在/不存在和价/半核心轨道的相对论收缩/膨胀。每当这些轨道类型在空间上重合时,共价键配对就会被半核电子的泡利排斥减弱,而 CSB 会接管。因此,对于拥有 (n-1)s2(n-1)p6 半核的 3-10 组,这种空间重合在 3d 过渡金属处最大,从而形成电荷转移 MM 键。然而,在第 11 和 12 组中,相对论效应使第三系列中的空间重合最大化,其中 5d10 核心接近价 6s 轨道,并且各自的泡利排斥产生具有 CSB 特征的 MM 键。这些考虑为过渡元素时期的 MM 键合创建了一个通用范式,
更新日期:2020-06-23
中文翻译:

关于过渡金属配合物中金属-金属键的共价与电荷转移性质:统一理解
我们在此通过使用 ab-initio 价键理论,提出了跨 TM 元素周期的过渡金属 (TM) 配合物中金属-金属 (MM) 键合性质的一般概念。计算揭示了双重趋势:对于第 7 组和第 9 组中的 MM 键,3d 系列形成电荷转移键 (CSB),而在向下移动到 5d 系列时,这些键逐渐变成共价键。相比之下,具有填充 d 轨道(第 11 和 12 族)的金属的 MM 键表现相反;最初 MM 键是共价键,但在元素周期表中向下移动时,CSB 特征增加。这些趋势源于原子轨道的径向分布函数,它决定了价轨道相对于填充的半核轨道的紧凑性。衡量这种紧凑性的关键因素是价轨道中径向节点的存在/不存在和价/半核心轨道的相对论收缩/膨胀。每当这些轨道类型在空间上重合时,共价键配对就会被半核电子的泡利排斥减弱,而 CSB 会接管。因此,对于拥有 (n-1)s2(n-1)p6 半核的 3-10 组,这种空间重合在 3d 过渡金属处最大,从而形成电荷转移 MM 键。然而,在第 11 和 12 组中,相对论效应使第三系列中的空间重合最大化,其中 5d10 核心接近价 6s 轨道,并且各自的泡利排斥产生具有 CSB 特征的 MM 键。这些考虑为过渡元素时期的 MM 键合创建了一个通用范式,











































 京公网安备 11010802027423号
京公网安备 11010802027423号