当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fast Proton Transfer and Hydrogen Evolution Reactivity Mediated by [Co13C2(CO)24]4-
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-23 , DOI: 10.1021/jacs.0c04034 Cody R Carr 1 , Atefeh Taheri 1 , Louise A Berben 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-23 , DOI: 10.1021/jacs.0c04034 Cody R Carr 1 , Atefeh Taheri 1 , Louise A Berben 1
Affiliation
|
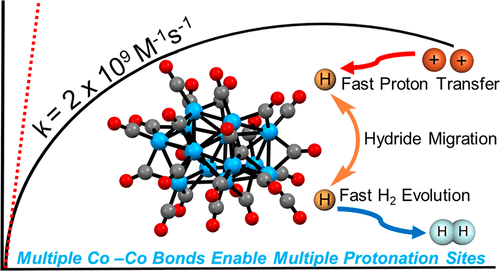
A common approach to speeding up proton transfer (PT) by molecular catalysts is manipulation of the secondary coordina-tion sphere with proton relays and these enhance overall reaction rates by orders of magnitude. In contrast, heterogenous electrocatalysts have band structures that promote facile PT concerted with electron transfer (ET), known as the Volmer mechanism. Here, we show that [Co13C2(CO)24]4-, containing multiple Co-Co bonds to statistically enhance observed rates of PT, promotes PT on the order of 2.3 × 10^9 M-1s-1 which suggests a diffusion-limited rate.. The fast ET and PT chemistry is attributed to the delocalized electronic structure of [Co13C2(CO)24]4-. Electrochemical characterization of [Co13C2(CO)24]4- in the presence and absence of protons reveals ET kinetics and diffusion behavior similar to other small clusters such as nanomaterials and fullerenes.
中文翻译:

[Co13C2(CO)24]4-介导的快速质子转移和析氢反应
通过分子催化剂加速质子转移 (PT) 的一种常见方法是使用质子中继操纵二级配位球,这些将整体反应速率提高了几个数量级。相比之下,异质电催化剂的能带结构促进了与电子转移 (ET) 协调的简易 PT,称为 Volmer 机制。在这里,我们表明 [Co13C2(CO)24]4- 包含多个 Co-Co 键以在统计上提高观察到的 PT 速率,促进 PT 的数量级为 2.3 × 10^9 M-1s-1,这表明扩散-有限的速率。快速的 ET 和 PT 化学归因于 [Co13C2(CO)24]4- 的离域电子结构。
更新日期:2020-06-23
中文翻译:

[Co13C2(CO)24]4-介导的快速质子转移和析氢反应
通过分子催化剂加速质子转移 (PT) 的一种常见方法是使用质子中继操纵二级配位球,这些将整体反应速率提高了几个数量级。相比之下,异质电催化剂的能带结构促进了与电子转移 (ET) 协调的简易 PT,称为 Volmer 机制。在这里,我们表明 [Co13C2(CO)24]4- 包含多个 Co-Co 键以在统计上提高观察到的 PT 速率,促进 PT 的数量级为 2.3 × 10^9 M-1s-1,这表明扩散-有限的速率。快速的 ET 和 PT 化学归因于 [Co13C2(CO)24]4- 的离域电子结构。










































 京公网安备 11010802027423号
京公网安备 11010802027423号