当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of (−)-Picrotoxinin by Late-Stage Strong Bond Activation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-23 , DOI: 10.1021/jacs.0c05042 Steven W M Crossley 1 , Guanghu Tong 1 , Michael J Lambrecht 1 , Hannah E Burdge 1 , Ryan A Shenvi 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-06-23 , DOI: 10.1021/jacs.0c05042 Steven W M Crossley 1 , Guanghu Tong 1 , Michael J Lambrecht 1 , Hannah E Burdge 1 , Ryan A Shenvi 1
Affiliation
|
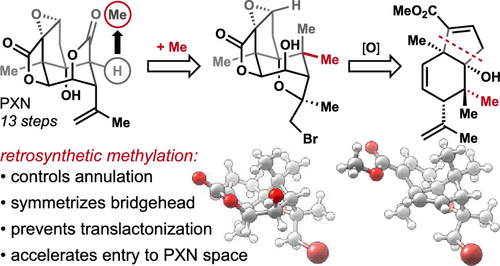
We report a concise, stereocontrolled synthesis of the neurotoxic sesquiterpenoid (-)-picrotoxinin (1, PXN). The brevity of the route is due to regio- and stereoselective formation of the [4.3.0] bicyclic core by incorporation of a symmetrizing geminal dimethyl group at C5. Dimethylation then enables selective C-O bond formation in multiple intermediates. A series of strong bond (C-C and C-H) cleavages convert the C5 gem-dimethyl group to the C15 lactone of PXN.
中文翻译:

通过后期强键激活合成 (−)-印防己毒素
我们报告了神经毒性倍半萜类化合物 (-)-印防己毒素 (1, PXN) 的简洁、立体控制合成。该路线的简洁性是由于通过在 C5 处掺入对称的偕二甲基基团来区域和立体选择性地形成[4.3.0]双环核心。二甲基化可以在多个中间体中选择性形成 CO 键。一系列强键(CC 和 CH)裂解将 C5 偕二甲基基团转化为 PXN 的 C15 内酯。
更新日期:2020-06-23
中文翻译:

通过后期强键激活合成 (−)-印防己毒素
我们报告了神经毒性倍半萜类化合物 (-)-印防己毒素 (1, PXN) 的简洁、立体控制合成。该路线的简洁性是由于通过在 C5 处掺入对称的偕二甲基基团来区域和立体选择性地形成[4.3.0]双环核心。二甲基化可以在多个中间体中选择性形成 CO 键。一系列强键(CC 和 CH)裂解将 C5 偕二甲基基团转化为 PXN 的 C15 内酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号