当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Cyclooctane and l-Tryptophan on Hydrate Formation from an Equimolar CO2–CH4 Gas Mixture Employing a Horizontal-Tray Packed Bed Reactor
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.energyfuels.0c01511 Namrata Gaikwad 1, 2 , Gaurav Bhattacharjee 2 , Omkar S. Kushwaha 1 , Jitendra S. Sangwai 3 , Praveen Linga 2 , Rajnish Kumar 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-06-23 , DOI: 10.1021/acs.energyfuels.0c01511 Namrata Gaikwad 1, 2 , Gaurav Bhattacharjee 2 , Omkar S. Kushwaha 1 , Jitendra S. Sangwai 3 , Praveen Linga 2 , Rajnish Kumar 1
Affiliation
|
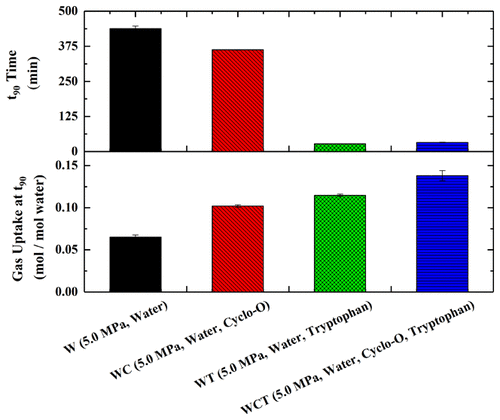
A fundamental study on hydrate formation from an equimolar CO2–CH4 gas mixture has been carried out with two focal points: accelerating the kinetics of hydrate formation and enhancing the gas separation efficiency of the process. To this effect, the impact of inducing different hydrate structures from the same gas mixture by introducing suitable additives into the system has been investigated, and experiments are being carried out in a horizontal packed bed reactor at two different initial pressures, 3.5 and 5.0 MPa, to study the effect of driving forces on the kinetics of hydrate formation and the separation efficiency of the process. sH hydrate former cyclooctane (Cyclo-O) induces rapid nucleation of hydrate and also yields significant gas uptake in hydrates, 29.55% higher compared to the water system. This may be attributed to the simultaneous formation of sH and sI hydrates when Cyclo-O is present in the system. It was observed that the environmentally benign hydrophobic amino acid tryptophan in low concentration (1 wt %) can effectively accelerate the kinetics of hydrate formation, with 90% water to hydrate conversion being obtained within the first 30 min of hydrate formation. Further, the use of Cyclo-O and tryptophan together shows a synergistic effect, resulting in the highest gas uptake among all the systems studied. Although the problem of slow kinetics of hydrate formation from CO2–CH4 gas mixtures has been satisfactorily solved through this work, there are still significant strides that need to be made toward improving the separation efficiency of the process. The formation of the mixed hydrate is unable to return a satisfactorily high efficiency for gas separation.
中文翻译:

环辛烷和l-色氨酸对采用卧式板式床反应器的等摩尔CO 2 -CH 4气体混合物中水合物形成的影响
从等摩尔CO 2 -CH 4形成水合物的基础研究进行气体混合有两个重点:加快水合物形成的动力学并提高该方法的气体分离效率。为此,已经研究了通过向系统中引入合适的添加剂从同一气体混合物中诱导出不同水合物结构的影响,并在卧式填充床反应器中以两种不同的初始压力(3.5和5.0 MPa)进行了实验,研究驱动力对水合物形成动力学和过程分离效率的影响。sH水合物的前环辛烷(Cyclo-O)诱导水合物快速成核,并且还产生大量的水合物气体吸收,比水系统高29.55%。这可能归因于系统中存在Cyclo-O时同时形成sH和sI水合物。观察到低浓度(1重量%)的环境友好的疏水性氨基酸色氨酸可以有效地加速水合物形成的动力学,在水合物形成的前30分钟内获得了90%的水到水合物的转化。此外,同时使用Cyclo-O和色氨酸显示出协同作用,从而在所有研究的系统中产生最高的气体吸收量。尽管从CO生成水合物动力学缓慢的问题 在水合物形成的前30分钟内获得了90%的水到水合物的转化率。此外,同时使用Cyclo-O和色氨酸显示出协同作用,从而在所有研究的系统中产生最高的气体吸收量。尽管从CO生成水合物动力学缓慢的问题 在水合物形成的前30分钟内获得了90%的水到水合物的转化率。此外,同时使用Cyclo-O和色氨酸表现出协同作用,从而在所有研究的系统中产生最高的气体吸收量。尽管从CO生成水合物动力学缓慢的问题通过这项工作已令人满意地解决了2 – CH 4气体混合物的问题,在提高工艺分离效率方面仍需要取得重大进展。混合水合物的形成不能使气体分离返回令人满意的高效率。
更新日期:2020-08-20
中文翻译:

环辛烷和l-色氨酸对采用卧式板式床反应器的等摩尔CO 2 -CH 4气体混合物中水合物形成的影响
从等摩尔CO 2 -CH 4形成水合物的基础研究进行气体混合有两个重点:加快水合物形成的动力学并提高该方法的气体分离效率。为此,已经研究了通过向系统中引入合适的添加剂从同一气体混合物中诱导出不同水合物结构的影响,并在卧式填充床反应器中以两种不同的初始压力(3.5和5.0 MPa)进行了实验,研究驱动力对水合物形成动力学和过程分离效率的影响。sH水合物的前环辛烷(Cyclo-O)诱导水合物快速成核,并且还产生大量的水合物气体吸收,比水系统高29.55%。这可能归因于系统中存在Cyclo-O时同时形成sH和sI水合物。观察到低浓度(1重量%)的环境友好的疏水性氨基酸色氨酸可以有效地加速水合物形成的动力学,在水合物形成的前30分钟内获得了90%的水到水合物的转化。此外,同时使用Cyclo-O和色氨酸显示出协同作用,从而在所有研究的系统中产生最高的气体吸收量。尽管从CO生成水合物动力学缓慢的问题 在水合物形成的前30分钟内获得了90%的水到水合物的转化率。此外,同时使用Cyclo-O和色氨酸显示出协同作用,从而在所有研究的系统中产生最高的气体吸收量。尽管从CO生成水合物动力学缓慢的问题 在水合物形成的前30分钟内获得了90%的水到水合物的转化率。此外,同时使用Cyclo-O和色氨酸表现出协同作用,从而在所有研究的系统中产生最高的气体吸收量。尽管从CO生成水合物动力学缓慢的问题通过这项工作已令人满意地解决了2 – CH 4气体混合物的问题,在提高工艺分离效率方面仍需要取得重大进展。混合水合物的形成不能使气体分离返回令人满意的高效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号