当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biocatalytic, Stereoselective Deuteration of α-Amino Acids and Methyl Esters
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-23 , DOI: 10.1021/acscatal.0c01885 Stephanie W Chun 1, 2 , Alison R H Narayan 1, 2, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-06-23 , DOI: 10.1021/acscatal.0c01885 Stephanie W Chun 1, 2 , Alison R H Narayan 1, 2, 3
Affiliation
|
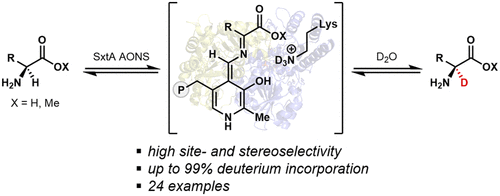
α-2H Amino acids are valuable precursors toward labeled pharmaceutical agents and tools for studying biological systems; however, these molecules are costly to purchase and challenging to synthesize in a site- and stereoselective manner. Here, we show that an α-oxoamine synthase that evolved for saxitoxin biosynthesis, SxtA AONS, is capable of producing a range of α-2H amino acids and esters site- and stereoselectively using D2O as the deuterium source. Additionally, we demonstrate the utility of this operationally simple reaction on preparative-scale in the stereoselective chemoenzymatic synthesis of a deuterated analogue of safinamide, a drug used to treat Parkinson’s disease.
中文翻译:

α-氨基酸和甲酯的生物催化、立体选择性氘化
α- 2 H 氨基酸是标记药剂和生物系统研究工具的宝贵前体;然而,这些分子的购买成本很高,而且以位点和立体选择性的方式合成具有挑战性。在这里,我们展示了为石房蛤毒素生物合成而进化的 α-氧代胺合酶 SxtA AONS,能够使用 D 2 O 作为氘源以立体选择性的方式产生一系列 α- 2 H 氨基酸和酯。此外,我们证明了这种操作简单的反应在制备规模的立体选择性化学酶促合成 safinamide 的氘代类似物(一种用于治疗帕金森病的药物)中的效用。
更新日期:2020-07-02
中文翻译:

α-氨基酸和甲酯的生物催化、立体选择性氘化
α- 2 H 氨基酸是标记药剂和生物系统研究工具的宝贵前体;然而,这些分子的购买成本很高,而且以位点和立体选择性的方式合成具有挑战性。在这里,我们展示了为石房蛤毒素生物合成而进化的 α-氧代胺合酶 SxtA AONS,能够使用 D 2 O 作为氘源以立体选择性的方式产生一系列 α- 2 H 氨基酸和酯。此外,我们证明了这种操作简单的反应在制备规模的立体选择性化学酶促合成 safinamide 的氘代类似物(一种用于治疗帕金森病的药物)中的效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号