当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Diversity‐Oriented Synthesis of Orthogonally Protected Cyclic Amino Acid Derivatives with Multiple Stereogenic Centers
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-06-23 , DOI: 10.1002/hlca.202000090 Melinda Nonn 1, 2, 3 , Attila M. Remete 1, 2 , Loránd Kiss 1, 2
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2020-06-23 , DOI: 10.1002/hlca.202000090 Melinda Nonn 1, 2, 3 , Attila M. Remete 1, 2 , Loránd Kiss 1, 2
Affiliation
|
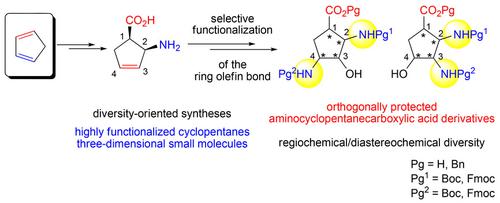
The synthesis of three‐dimensional cyclopentane amino acid derivatives with multiple stereocenters and with high regiochemical and diastereochemical diversity has been achieved starting from cyclopentadiene‐derived β‐aminocyclopentenecarboxylic acid. The small‐molecular design was based on stereo‐ and regiocontrolled functionalization of the starting cyclopentene β‐amino acid through stereoselective oxirane formation/regioselective oxirane opening and resulted in regio‐ and diastereoisomers of novel orthogonally protected aminocyclopentanecarboxylates.
中文翻译:

面向结构多样性的具有多个立体中心的正交保护的环状氨基酸衍生物的合成
从环戊二烯衍生的β-氨基环戊烯羧酸开始,已经实现了具有多个立体中心,具有较高的区域化学和非对映化学多样性的三维环戊烷氨基酸衍生物的合成。小分子设计基于起始环戊烯β-氨基酸通过立体选择性环氧乙烷的形成/区域选择性环氧乙烷的打开而进行的立体和区域控制官能化,并导致了新型正交保护的氨基环戊烷羧酸盐的区域和非对映异构体。
更新日期:2020-06-23
中文翻译:

面向结构多样性的具有多个立体中心的正交保护的环状氨基酸衍生物的合成
从环戊二烯衍生的β-氨基环戊烯羧酸开始,已经实现了具有多个立体中心,具有较高的区域化学和非对映化学多样性的三维环戊烷氨基酸衍生物的合成。小分子设计基于起始环戊烯β-氨基酸通过立体选择性环氧乙烷的形成/区域选择性环氧乙烷的打开而进行的立体和区域控制官能化,并导致了新型正交保护的氨基环戊烷羧酸盐的区域和非对映异构体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号