当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical Nonacidic N‐Nitrosation/N‐Nitration of Secondary Amines through a Biradical Coupling Reaction
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-06-22 , DOI: 10.1002/adsc.202000267 Ji‐Ping Zhao 1 , Lu‐jia Ding 1 , Peng‐Cheng Wang 1 , Ying Liu 1 , Min‐Jun Huang 1 , Xin‐Li Zhou 1 , Ming Lu 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-06-22 , DOI: 10.1002/adsc.202000267 Ji‐Ping Zhao 1 , Lu‐jia Ding 1 , Peng‐Cheng Wang 1 , Ying Liu 1 , Min‐Jun Huang 1 , Xin‐Li Zhou 1 , Ming Lu 1
Affiliation
|
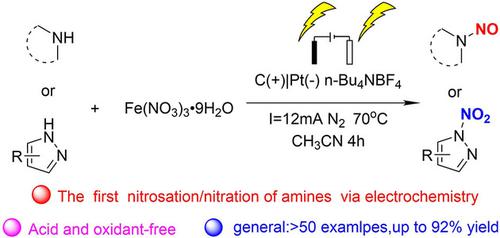
An acid‐free N‐nitrosation/nitration of the N−H bonds in secondary amines with Fe(NO3)3 ⋅ 9H2O as the nitroso/nitro source through an electrocatalyzed radical coupling reaction was developed. Cyclic aliphatic amines and N‐heteroaromatic compounds were N‐nitrosated and N‐nitrated, respectively, under mild conditions. Control and competition experiments, as well as kinetic studies, demonstrate that N‐nitrosation and N‐nitration involve two different radical reaction pathways involving N+ and N. radicals. Moreover, the electrocatalysis method enables the preferential activation of the N−H bond over the electrode and thus provides high selectivity for specific N atoms. Finally, this strategy exhibits a broad scope and provides a green and straightforward approach to generate useful N‐nitroso/nitro compounds in good yields.
中文翻译:

通过双自由基偶联反应对仲胺进行电化学非酸性N-亚硝化/ N-硝化
在仲胺的N-H键为Fe的游离酸-N-亚硝化/硝化(NO 3)3 ⋅9H 2 O为通过电催化自由基偶合反应的亚硝基/硝基源被开发。环状脂肪胺和N-杂芳族化合物分别在温和的条件下进行N-亚硝化和N-硝化。控制和竞争实验,以及动力学研究,证明了的N-亚硝化和N-硝化涉及涉及Ñ两个不同的自由基反应途径+和N 。部首。此外,电催化方法使电极上的NH键优先活化,因此对特定N原子具有高选择性。最后,该策略具有广泛的应用范围,并提供了一种绿色且直接的方法来以高收率生成有用的N-亚硝基/硝基化合物。
更新日期:2020-06-22
中文翻译:

通过双自由基偶联反应对仲胺进行电化学非酸性N-亚硝化/ N-硝化
在仲胺的N-H键为Fe的游离酸-N-亚硝化/硝化(NO 3)3 ⋅9H 2 O为通过电催化自由基偶合反应的亚硝基/硝基源被开发。环状脂肪胺和N-杂芳族化合物分别在温和的条件下进行N-亚硝化和N-硝化。控制和竞争实验,以及动力学研究,证明了的N-亚硝化和N-硝化涉及涉及Ñ两个不同的自由基反应途径+和N 。部首。此外,电催化方法使电极上的NH键优先活化,因此对特定N原子具有高选择性。最后,该策略具有广泛的应用范围,并提供了一种绿色且直接的方法来以高收率生成有用的N-亚硝基/硝基化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号