当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, biological evaluation, and docking study of a series of 1,4‐disubstituted 1,2,3‐triazole derivatives with an indole‐triazole‐peptide conjugate
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-06-23 , DOI: 10.1002/jhet.4020 Srinivas Suryapeta 1 , Neeraja Papigani 2 , Venkanna Banothu 3 , Pramod Kumar Dubey 4 , Khagga Mukkanti 5 , Sarbani Pal 6
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-06-23 , DOI: 10.1002/jhet.4020 Srinivas Suryapeta 1 , Neeraja Papigani 2 , Venkanna Banothu 3 , Pramod Kumar Dubey 4 , Khagga Mukkanti 5 , Sarbani Pal 6
Affiliation
|
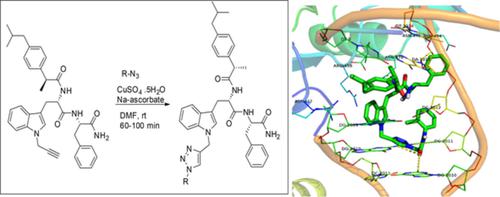
A series of new compounds containing an indole‐triazole‐ peptide conjugate were designed as potential agents possessing the dual anti‐bacterial and anticancer activities. Accordingly, 20 compounds were prepared via a multi‐step synthesis involving the copper‐catalyzed azide‐alkyne cycloaddition (CuAAC) as a key step in moderate to high yield. All the synthesized compounds were purified by chromatographic techniques and characterized by IR, 1H and 13C NMR and mass spectral data. The synthesized derivatives were screened for their antimicrobial activities against one gram‐positive (Staphylococcus aureus ) and three gram‐negative (Escherichia coli , Klebsiella pneumonia , and Proteus vulgaris ) bacteria using an agar‐well diffusion method. Most of the compounds showed moderate to reasonable antibacterial activities especially the compound 9e that showed good activities against all the strains. The potential of DNA gyrase inhibitory activity of this compound was assessed by using molecular docking studies in silico carried out using Autodock Vina software. The low ΔGbind value (−9.4 Kcal/mol) of compound 9e suggested its good interactions with the target protein in silico. The cytotoxic activities of some of the compounds synthesized were evaluated via a MTT assay using the human lung cancer cell line A549. Several compounds showed promising activities among which compound 9b , 9k, and 9e showed low IC50 values.
中文翻译:

一系列1,4-二取代1,2,3-三唑与吲哚-三唑-肽共轭物的合成,生物学评估和对接研究
设计了一系列含有吲哚-三唑-肽共轭物的新化合物,作为具有双重抗菌和抗癌活性的潜在药物。因此,通过多步合成制备了20种化合物,其中包括铜催化的叠氮化物-炔烃环加成(CuAAC),这是中高收率的关键步骤。所有合成的化合物均通过色谱技术纯化,并通过IR,1 H和13 C NMR和质谱数据表征。筛选了合成衍生物对一种革兰氏阳性菌(金黄色葡萄球菌)和三种革兰氏阴性菌(大肠杆菌,克雷伯菌肺炎和普通变形杆菌(Proteus vulgaris)细菌采用琼脂井扩散法。大多数化合物显示出中等至合理的抗菌活性,尤其是化合物9e对所有菌株均表现出良好的活性。通过使用Autodock Vina软件在计算机上进行的分子对接研究,评估了该化合物的DNA促旋酶抑制活性的潜力。化合物9e的低ΔG结合值(-9.4 Kcal / mol)表明其与靶蛋白在计算机上具有良好的相互作用。使用人肺癌细胞系A549通过MTT分析评估了某些合成化合物的细胞毒活性。几种化合物显示出令人鼓舞的活性,其中化合物9b,9k和9e显示出较低的IC 50值。
更新日期:2020-08-08
中文翻译:

一系列1,4-二取代1,2,3-三唑与吲哚-三唑-肽共轭物的合成,生物学评估和对接研究
设计了一系列含有吲哚-三唑-肽共轭物的新化合物,作为具有双重抗菌和抗癌活性的潜在药物。因此,通过多步合成制备了20种化合物,其中包括铜催化的叠氮化物-炔烃环加成(CuAAC),这是中高收率的关键步骤。所有合成的化合物均通过色谱技术纯化,并通过IR,1 H和13 C NMR和质谱数据表征。筛选了合成衍生物对一种革兰氏阳性菌(金黄色葡萄球菌)和三种革兰氏阴性菌(大肠杆菌,克雷伯菌肺炎和普通变形杆菌(Proteus vulgaris)细菌采用琼脂井扩散法。大多数化合物显示出中等至合理的抗菌活性,尤其是化合物9e对所有菌株均表现出良好的活性。通过使用Autodock Vina软件在计算机上进行的分子对接研究,评估了该化合物的DNA促旋酶抑制活性的潜力。化合物9e的低ΔG结合值(-9.4 Kcal / mol)表明其与靶蛋白在计算机上具有良好的相互作用。使用人肺癌细胞系A549通过MTT分析评估了某些合成化合物的细胞毒活性。几种化合物显示出令人鼓舞的活性,其中化合物9b,9k和9e显示出较低的IC 50值。









































 京公网安备 11010802027423号
京公网安备 11010802027423号