当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple Fused Anthracenes as Helical Polycyclic Aromatic Hydrocarbon Motif for Chiroptical Performance Enhancement.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-06-23 , DOI: 10.1002/asia.202000394 Kei Fujise 1 , Eiji Tsurumaki 1 , Gaku Fukuhara 1, 2 , Nobuyuki Hara 3 , Yoshitane Imai 3 , Shinji Toyota 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-06-23 , DOI: 10.1002/asia.202000394 Kei Fujise 1 , Eiji Tsurumaki 1 , Gaku Fukuhara 1, 2 , Nobuyuki Hara 3 , Yoshitane Imai 3 , Shinji Toyota 1
Affiliation
|
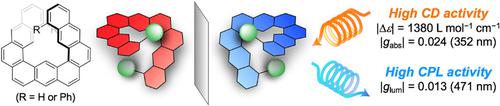
Polycyclic aromatic hydrocarbons consisting of three fused anthracene units were designed as new π‐conjugated compounds having helical structures. These expanded helicenes were synthesized by Pt‐catalyzed cycloisomerization of the corresponding ethynyl‐substituted precursors. The nonplanar and helical structure was confirmed by X‐ray analysis and DFT calculations, and the barrier to helical inversion was estimated to be 34 kJ mol−1. The enantiomers of the diphenyl derivative were successfully resolved by chiral HPLC. Enantiopure samples showed good chiroptical performance in the CD (|Δϵ| 1380 L mol−1 cm−1) and CPL (|glum| 0.013) spectra, and these values were considerably large for simple organic molecules. The unique chiroptical properties are discussed on the basis of the molecular structure and the electronic state with the aid of time‐dependent DFT calculations.
中文翻译:

多种熔融蒽作为螺旋多环芳烃基团,可增强手性。
由三个稠合蒽单元组成的多环芳烃被设计为具有螺旋结构的新型π共轭化合物。这些膨胀的螺旋烯是通过相应乙炔基取代的前体的Pt催化环异构化合成的。通过X射线分析和DFT计算证实了非平面螺旋结构,并且螺旋反转的势垒估计为34 kJ mol -1。通过手性HPLC成功拆分了二苯基衍生物的对映异构体。对映纯样品在CD(| Δϵ | 1380 L mol -1 cm -1)和CPL(| g lum| 0.013)光谱,而对于简单的有机分子,这些值相当大。在分子结构和电子态的基础上,借助依赖于时间的DFT计算,讨论了独特的手性。
更新日期:2020-08-18
中文翻译:

多种熔融蒽作为螺旋多环芳烃基团,可增强手性。
由三个稠合蒽单元组成的多环芳烃被设计为具有螺旋结构的新型π共轭化合物。这些膨胀的螺旋烯是通过相应乙炔基取代的前体的Pt催化环异构化合成的。通过X射线分析和DFT计算证实了非平面螺旋结构,并且螺旋反转的势垒估计为34 kJ mol -1。通过手性HPLC成功拆分了二苯基衍生物的对映异构体。对映纯样品在CD(| Δϵ | 1380 L mol -1 cm -1)和CPL(| g lum| 0.013)光谱,而对于简单的有机分子,这些值相当大。在分子结构和电子态的基础上,借助依赖于时间的DFT计算,讨论了独特的手性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号