当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Why Boron Nitride is such a Selective Catalyst for the Oxidative Dehydrogenation of Propane.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-06-22 , DOI: 10.1002/anie.202003695 Juan M Venegas 1, 2 , Zisheng Zhang 3 , Theodore O Agbi 1 , William P McDermott 4 , Anastassia Alexandrova 3 , Ive Hermans 1, 4
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-06-22 , DOI: 10.1002/anie.202003695 Juan M Venegas 1, 2 , Zisheng Zhang 3 , Theodore O Agbi 1 , William P McDermott 4 , Anastassia Alexandrova 3 , Ive Hermans 1, 4
Affiliation
|
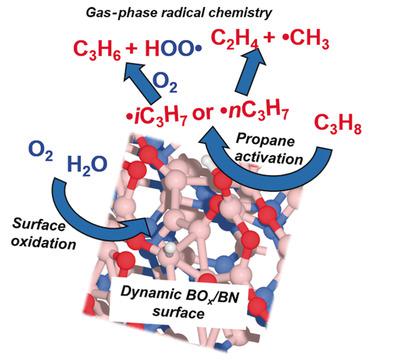
Boron‐containing materials, and in particular boron nitride, have recently been identified as highly selective catalysts for the oxidative dehydrogenation of alkanes such as propane. To date, no mechanism exists that can explain both the unprecedented selectivity, the observed surface oxyfunctionalization, and the peculiar kinetic features of this reaction. We combine catalytic activity measurements with quantum chemical calculations to put forward a bold new hypothesis. We argue that the remarkable product distribution can be rationalized by a combination of surface‐mediated formation of radicals over metastable sites, and their sequential propagation in the gas phase. Based on known radical propagation steps, we quantitatively describe the oxygen pressure‐dependent relative formation of the main product propylene and by‐product ethylene. Free radical intermediates most likely differentiate this catalytic system from less selective vanadium‐based catalysts.
中文翻译:

为什么氮化硼是这样一种丙烷氧化脱氢的选择性催化剂。
含硼材料,特别是氮化硼,最近被确定为烷烃(如丙烷)氧化脱氢的高选择性催化剂。迄今为止,还没有机制可以解释前所未有的选择性,观察到的表面氧官能化以及该反应的特殊动力学特征。我们将催化活性测量与量子化学计算相结合,提出了一个大胆的新假设。我们认为,显着的产物分布可以通过将表面介导的自由基在亚稳位上的形成以及它们在气相中的顺序传播相结合来合理化。基于已知的自由基传播步骤,我们定量描述了主要产品丙烯和副产品乙烯的氧压依赖性相对形成。
更新日期:2020-06-22
中文翻译:

为什么氮化硼是这样一种丙烷氧化脱氢的选择性催化剂。
含硼材料,特别是氮化硼,最近被确定为烷烃(如丙烷)氧化脱氢的高选择性催化剂。迄今为止,还没有机制可以解释前所未有的选择性,观察到的表面氧官能化以及该反应的特殊动力学特征。我们将催化活性测量与量子化学计算相结合,提出了一个大胆的新假设。我们认为,显着的产物分布可以通过将表面介导的自由基在亚稳位上的形成以及它们在气相中的顺序传播相结合来合理化。基于已知的自由基传播步骤,我们定量描述了主要产品丙烯和副产品乙烯的氧压依赖性相对形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号