Structure ( IF 4.4 ) Pub Date : 2020-06-23 , DOI: 10.1016/j.str.2020.06.002 Kyungjin Min 1 , Hye-Jin Yoon 1 , Ji Young Park 2 , Mithu Baidya 3 , Hemlata Dwivedi-Agnihotri 3 , Jagannath Maharana 3 , Madhu Chaturvedi 3 , Ka Young Chung 2 , Arun K Shukla 3 , Hyung Ho Lee 1
|
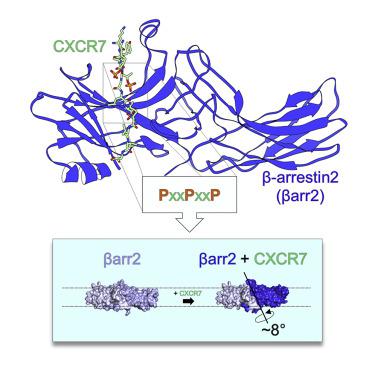
β-Arrestins (βarrs) critically regulate G-protein-coupled receptor (GPCR) signaling and trafficking. βarrs have two isoforms, βarr1 and βarr2. Receptor phosphorylation is a key determinant for the binding of βarrs, and understanding the intricate details of receptor-βarr interaction is the next frontier in GPCR structural biology. The high-resolution structure of active βarr1 in complex with a phosphopeptide derived from GPCR has been revealed, but that of βarr2 remains elusive. Here, we present a 2.3-Å crystal structure of βarr2 in complex with a phosphopeptide (C7pp) derived from the carboxyl terminus of CXCR7. The structural analysis of C7pp-bound βarr2 reveals key differences from the previously determined active conformation of βarr1. One of the key differences is that C7pp-bound βarr2 shows a relatively small inter-domain rotation. Antibody-fragment-based conformational sensor and hydrogen/deuterium exchange experiments further corroborated the structural features of βarr2 and suggested that βarr2 adopts a range of inter-domain rotations.
中文翻译:

β-Arrestin 2 与 CXCR7 磷酸肽复合物的晶体结构。
β-Arrestins (βarrs) 严格调节 G 蛋白偶联受体 (GPCR) 信号传导和运输。βarr 有两种异构体,βarr1 和 βarr2。受体磷酸化是 βarr 结合的关键决定因素,了解受体-βarr 相互作用的复杂细节是 GPCR 结构生物学的下一个前沿。已经揭示了与源自 GPCR 的磷酸肽复合的活性 βarr1 的高分辨率结构,但 βarr2 的高分辨率结构仍然难以捉摸。在这里,我们展示了一个 2.3-Å 晶体结构的 βarr2 与源自 CXCR7 羧基末端的磷酸肽 (C7pp) 复合。C7pp结合的βarr2的结构分析揭示了与先前确定的βarr1活性构象的关键差异。关键区别之一是 C7pp 结合的 βarr2 显示出相对较小的域间旋转。











































 京公网安备 11010802027423号
京公网安备 11010802027423号