Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2020-06-23 , DOI: 10.1016/j.micromeso.2020.110398 Huiling Tang , Yuehua Gou , Zhengdong Yan , Qingqing Hu , Fumin Zhang , Qiang Xiao , Yijun Zhong , Weidong Zhu
|
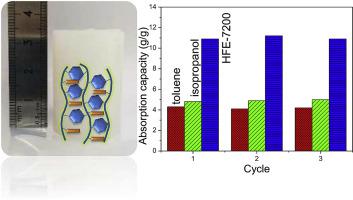
Functionalized porous polymeric materials have a good potential for adsorption application owing to their abundant porosity, group tunability and chemical stability. Up to now, few studies have been reported on the fluorous porous polymers. Here, we report a solvothermal synthesis of fluorous porous poly methacrylate-divinyl benzene (FPMD) materials through a copolymerization of 2-(perfluorohexyl)ethyl methacrylate (C6Rf) and divinylbenzene (DVB) in an autoclave. Various techniques, such as nitrogen adsorption-desorption, Fourier-transform infrared spectrometer (FTIR), scanning electron microscope (SEM), thermogravimetric analyzer (TG), X-ray photoelectron spectroscopy (XPS) and so on have been used to characterize the structure and morphology of the synthesized FPMD samples. It is found that C6Rf could copolymerize with DVB to form porous networks through a solvothermal reaction. The molar ratio of C6Rf and DVB as well as the solvent are closely related to the porosity of the FPMD. The porosity descends and even disappears when increasing the molar ratio of C6Rf/DVB to 1 in case of ethyl acetate as the solvent. While a fluorous solvent of hydrofluoroether (HFE-7300) is used, a FPMD with porous structure can be obtained at the same ratio. The fluorous solvent may favor the stretch of the fluorocarbon chains of C6Rf and thus produce porosity during the copolymerization. HFE-7200 with a lower boiling point has also been used as the fluorous solvent, finding that the pore structure of FPMD transforms to a typical bottle-shape mesopore structure. Pure solvent absorption tests indicate that the adsorption capacities to toluene, isopropanol and hydrofluoroether (HFE-7200) are 4.7, 6.2 and 7.8 ml/g, respectively, on the optimized FPMD material. The absorption capacity of HFE-7200 is higher than that of toluene and isopropanol, illustrating the fluorophilicity of the fluoridated porous materials.
中文翻译:

甲基丙烯酸2-(全氟己基)乙酯与二乙烯基苯的共聚反应成为氟类多孔聚合物材料的亲油性吸收剂
功能化的多孔聚合物材料具有丰富的孔隙率,基团可调性和化学稳定性,因此具有良好的吸附应用潜力。迄今为止,关于氟多孔聚合物的报道很少。在这里,我们报告了通过热共聚对氟多孔聚甲基丙烯酸甲酯-二乙烯基苯(FPMD)材料进行溶剂热合成。高压釜中的甲基丙烯酸2-(全氟己基)乙酯(C6Rf)和二乙烯基苯(DVB)。氮吸附-解吸,傅立叶变换红外光谱仪(FTIR),扫描电子显微镜(SEM),热重分析仪(TG),X射线光电子能谱(XPS)等各种技术已用于表征结构合成的FPMD样品的形态和形态。发现C6Rf可通过溶剂热反应与DVB共聚形成多孔网络。C6Rf和DVB以及溶剂的摩尔比与FPMD的孔隙率密切相关。在以乙酸乙酯为溶剂的情况下,当将C6Rf / DVB的摩尔比提高至1时,孔隙率降低甚至消失。使用氢氟醚的氟溶剂(HFE-7300)时,可以相同的比例获得具有多孔结构的FPMD。含氟溶剂可有利于C6Rf的碳氟链的拉伸,因此在共聚过程中产生孔隙。具有较低沸点的HFE-7200也已被用作氟溶剂,发现FPMD的孔结构转变为典型的瓶形中孔结构。纯溶剂吸收测试表明,在优化的FPMD材料上,甲苯,异丙醇和氢氟醚(HFE-7200)的吸附容量分别为4.7、6.2和7.8 ml / g。HFE-7200的吸收容量高于甲苯和异丙醇的吸收容量,说明了氟化多孔材料的亲氟性。含氟溶剂可有利于C6Rf的碳氟链的拉伸,因此在共聚过程中产生孔隙。具有较低沸点的HFE-7200也已被用作氟溶剂,发现FPMD的孔结构转变为典型的瓶形中孔结构。纯溶剂吸收测试表明,在优化的FPMD材料上,甲苯,异丙醇和氢氟醚(HFE-7200)的吸附容量分别为4.7、6.2和7.8 ml / g。HFE-7200的吸收容量高于甲苯和异丙醇的吸收容量,说明了氟化多孔材料的亲氟性。含氟溶剂可有利于C6Rf的碳氟链的拉伸,因此在共聚过程中产生孔隙。具有较低沸点的HFE-7200也已被用作氟溶剂,发现FPMD的孔结构转变为典型的瓶形中孔结构。纯溶剂吸收测试表明,在优化的FPMD材料上,甲苯,异丙醇和氢氟醚(HFE-7200)的吸附容量分别为4.7、6.2和7.8 ml / g。HFE-7200的吸收容量高于甲苯和异丙醇的吸收容量,说明了氟化多孔材料的亲氟性。纯溶剂吸收测试表明,在优化的FPMD材料上,甲苯,异丙醇和氢氟醚(HFE-7200)的吸附容量分别为4.7、6.2和7.8 ml / g。HFE-7200的吸收容量高于甲苯和异丙醇的吸收容量,说明了氟化多孔材料的亲氟性。纯溶剂吸收测试表明,在优化的FPMD材料上,甲苯,异丙醇和氢氟醚(HFE-7200)的吸附容量分别为4.7、6.2和7.8 ml / g。HFE-7200的吸收容量高于甲苯和异丙醇的吸收容量,说明了氟化多孔材料的亲氟性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号