Journal of Structural Biology ( IF 3 ) Pub Date : 2020-06-23 , DOI: 10.1016/j.jsb.2020.107553 Ashwin K Chetty 1 , Joel A Sexton 2 , Byung Hak Ha 2 , Benjamin E Turk 3 , Titus J Boggon 4
|
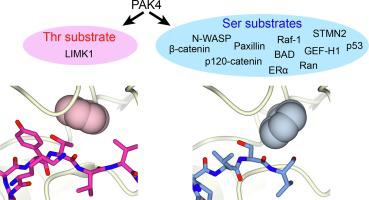
Many serine/threonine protein kinases discriminate between serine and threonine substrates as a filter to control signaling output. Among these, the p21-activated kinase (PAK) group strongly favors phosphorylation of Ser over Thr residues. PAK4, a group II PAK, almost exclusively phosphorylates its substrates on serine residues. The only well documented exception is LIM domain kinase 1 (LIMK1), which is phosphorylated on an activation loop threonine (Thr508) to promote its catalytic activity. To understand the molecular and kinetic basis for PAK4 substrate selectivity we compared its mode of recognition of LIMK1 (Thr508) with that of a known serine substrate, β-catenin (Ser675). We determined X-ray crystal structures of PAK4 in complex with synthetic peptides corresponding to its phosphorylation sites in LIMK1 and β-catenin to 1.9 Å and 2.2 Å resolution, respectively. We found that the PAK4 DFG + 1 residue, a key determinant of phosphoacceptor preference, adopts a sub-optimal orientation when bound to LIMK1 compared to β-catenin. In peptide kinase activity assays, we find that phosphoacceptor identity impacts catalytic efficiency but does not affect the Km value for both phosphorylation sites. Although catalytic efficiency of wild-type LIMK1 and β-catenin are equivalent, T508S mutation of LIMK1 creates a highly efficient substrate. These results suggest suboptimal phosphorylation of LIMK1 as a mechanism for controlling the dynamics of substrate phosphorylation by PAK4.
中文翻译:

p21 激活激酶 4 对生理磷酸化位点的识别。
许多丝氨酸/苏氨酸蛋白激酶区分丝氨酸和苏氨酸底物,作为控制信号输出的过滤器。其中,p21 激活激酶 (PAK) 组强烈支持 Ser 而非 Thr 残基的磷酸化。PAK4 是第 II 组 PAK,几乎只在丝氨酸残基上磷酸化其底物。唯一有据可查的例外是 LIM 结构域激酶 1 (LIMK1),它在激活环苏氨酸 (Thr508) 上被磷酸化以促进其催化活性。为了解 PAK4 底物选择性的分子和动力学基础,我们将其识别 LIMK1 (Thr508) 的模式与已知丝氨酸底物 β-连环蛋白 (Ser675) 的识别模式进行了比较。我们确定了与合成肽复合的 PAK4 的 X 射线晶体结构,该合成肽对应于其在 LIMK1 和 β-连环蛋白中的磷酸化位点,分别为 1.9 Å 和 2。分别为 2 Å 分辨率。我们发现,与 β-连环蛋白相比,PAK4 DFG + 1 残基是磷酸受体偏好的关键决定因素,当与 LIMK1 结合时采用次优方向。在肽激酶活性测定中,我们发现磷酸受体身份会影响催化效率但不会影响两个磷酸化位点的K m值。虽然野生型 LIMK1 和 β-catenin 的催化效率相当,但 LIMK1 的 T508S 突变产生了高效底物。这些结果表明 LIMK1 的次优磷酸化作为控制 PAK4 底物磷酸化动力学的机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号