Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2020-06-23 , DOI: 10.1016/j.jhazmat.2020.123292 Jianhua Qu 1 , Yuxin Wang 2 , Xue Tian 1 , Zhao Jiang 1 , Fengxia Deng 3 , Yue Tao 1 , Qun Jiang 1 , Lei Wang 1 , Ying Zhang 1
|
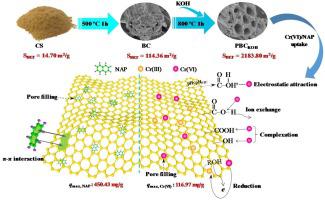
Herein, a high-performance porous biochar described as PBCKOH was successfully synthesized by two-step pyrolysis of corn straw with chemical activation of KOH, and was employed for the elimination of Cr(VI) and naphthalene (NAP) from water. Benefiting from KOH activation, the PBCKOH was found to possess huge specific surface area of 2183.80 m2/g and many well-developed micropores with average particle size of 2.75 nm and main pore diameters distribution from 1 to 2 nm. The PBCKOH presented an excellent adsorption performance with a theoretical monolayer uptake of 116.97 mg/g for Cr(VI) and a heterogeneous adsorption capacity of 450.43 mg/g for NAP. The uptake equilibrium was attained within about 120 min for Cr(VI), while about 180 min for NAP following avrami fractional-order model, revealing the existence of multiple kinetics during the adsorption. The thermodynamic results showed that the uptake of both Cr(VI) and NAP occurred spontaneously (-ΔG°), while in an endothermic nature for Cr(VI) (+ΔH°) and an exothermic characteristic for NAP (-ΔH°) with different randomness. Furthermore, the PBCKOH was believed to enhance the Cr(VI) adsorption mainly through the combination of electrostatic attraction, complexation, ion exchange and reduction action, while achieving the high NAP uptake by pore filling and π-π stacking interactions.



























 京公网安备 11010802027423号
京公网安备 11010802027423号