Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-06-23 , DOI: 10.1016/j.bbamem.2020.183404 Ujjayini Ghosh 1 , David P Weliky 1
|
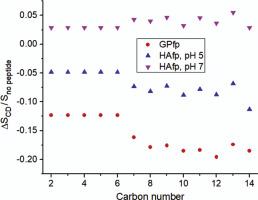
Enveloped viruses are surrounded by a membrane which is obtained from an infected host cell during budding. Infection of a new cell requires joining (fusion) of the virus and cell membranes. This process is mediated by a monotopic viral fusion protein with a large ectodomain outside the virus. The ectodomains of class I enveloped viruses have a N-terminal “fusion peptide” (fp) domain that is critical for fusion and binds to the cell membrane. In this study, 2H NMR spectra are analyzed for deuterated membrane with fp from either HIV gp41 (GP) or influenza hemagglutinin (HA) fusion proteins. In addition, the HAfp samples are studied at more fusogenic pH 5 and less fusogenic pH 7. GPfp adopts intermolecular antiparallel β sheet structure whereas HAfp is a monomeric helical hairpin. The data are obtained for a set of temperatures between 35 and 0 °C using DMPC-d54 lipid with perdeuterated acyl chains. The DMPC has liquid-crystalline (Lα) phase with disordered chains at higher temperature and rippled gel (Pβ′) or gel phase (Lβ′) with ordered chains at lower temperature. At given temperature T, the no peptide and HAfp, pH 7 samples exhibit similar spectral lineshapes. Spectral broadening with reduced temperature correlates with the transition from Lα to Pβ′ and then Lβ′ phases. At given T, the lineshapes are narrower for HAfp, pH 5 vs. no peptide and HAfp, pH 7 samples, and even narrower for the GPfp sample. These data support larger-amplitude fast (>105 Hz) lipid acyl chain motion for samples with fusogenic peptides, and peptide interference with chain ordering. The NMR data of the present paper correlate with insertion of these peptides into the hydrocarbon core of the membrane and support a significant fusion contribution from the resultant lipid acyl chain disorder, perhaps because of reduced barriers between the different membrane topologies in the fusion pathway. Membrane insertion and lipid perturbation appear common to both β sheet and helical hairpin peptides.
中文翻译:

2H 核磁共振波谱支持更大幅度的快速运动,并干扰含有 β 片状人类免疫缺陷病毒 gp41 融合肽或螺旋发夹流感病毒血凝素融合肽的膜在融合 pH 下的脂质链排序。
有包膜的病毒被一层膜包围,该膜是在出芽期间从受感染的宿主细胞中获得的。新细胞的感染需要病毒和细胞膜的结合(融合)。该过程由具有病毒外大胞外域的单位病毒融合蛋白介导。I 类包膜病毒的胞外域具有 N 端“融合肽”(fp) 域,该域对于融合至关重要并与细胞膜结合。在本研究中,2使用来自 HIV gp41 (GP) 或流感血凝素 (HA) 融合蛋白的 fp 分析氘化膜的 H NMR 谱。此外,HAfp 样品在更多的融合 pH 5 和更少的融合 pH 7 下进行研究。GPfp 采用分子间反平行 β 折叠结构,而 HAfp 是单体螺旋发夹。数据是在 35 到 0 °C 之间的一组温度下获得的,使用 DMPC-d54 脂质和全氘化酰基链。DMPC 具有液晶 (L α ) 相,在较高温度下具有无序链和波纹凝胶 (P β' ) 或凝胶相 (L β') 在较低温度下具有有序链。在给定温度 T 下,无肽和 Hafp、pH 7 样品表现出相似的光谱线形。随着温度降低,光谱展宽与从 L α到 P β'再到 L β'相的转变相关。在给定的 T 下,HAfp、pH 5 与无肽和 Hafp、pH 7 样品的线形更窄,而 GPfp 样品的线形更窄。这些数据支持较大幅度的快速(>10 5 Hz) 具有融合肽的样品的脂酰基链运动,以及肽对链排序的干扰。本论文的 NMR 数据与这些肽插入膜的碳氢化合物核心相关,并支持由此产生的脂酰基链紊乱对融合的重要贡献,这可能是因为融合途径中不同膜拓扑结构之间的障碍减少。膜插入和脂质扰动似乎对 β 折叠和螺旋发夹肽都很常见。











































 京公网安备 11010802027423号
京公网安备 11010802027423号