当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a telescoped synthesis of 4-(1H)-cyanoimidazole core accelerated by orthogonal reaction monitoring
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0re00234h Thomas C. Malig 1, 2, 3, 4 , Yichen Tan 5, 6, 7, 8 , Steven R. Wisniewski 5, 6, 7, 8 , Carolyn S. Higman 5, 6, 7, 8 , Ronald Carrasquillo-Flores 5, 6, 7, 8 , Adrian Ortiz 5, 6, 7, 8 , Geoffrey E. Purdum 5, 6, 7, 8 , Sergei Kolotuchin 5, 6, 7, 8 , Jason E. Hein 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0re00234h Thomas C. Malig 1, 2, 3, 4 , Yichen Tan 5, 6, 7, 8 , Steven R. Wisniewski 5, 6, 7, 8 , Carolyn S. Higman 5, 6, 7, 8 , Ronald Carrasquillo-Flores 5, 6, 7, 8 , Adrian Ortiz 5, 6, 7, 8 , Geoffrey E. Purdum 5, 6, 7, 8 , Sergei Kolotuchin 5, 6, 7, 8 , Jason E. Hein 1, 2, 3, 4
Affiliation
|
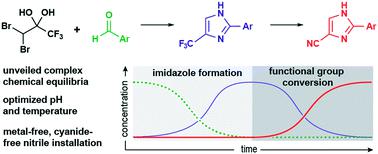
We have developed a convenient two-step, one-pot method for the facile synthesis of cyanoimidazoles. By integrating a suite of reaction-monitoring techniques, we were able to interrogate each transformation independently. We observed that formation of a key hemiaminal intermediate is complicated via many equilibrium processes, creating oligomers and eventually resulting in unproductive dimerization. DoE experiments indicated that despite the complex equilibria of this substrate, high conversion to the trifluoromethyl imidazole can still be achieved. We also report the effects of temperature, pH, and substrate concentrations on the rate and selectivity of the elimination cascade reaction converting the trifluoromethyl imidazole to the cyanoimidazole. By leveraging kinetic information gathered from each step independently, we report reaction conditions to achieve high yields of the cyanoimidazole from carbonyl-containing substrate directly in one pot.
中文翻译:

正交反应监测加速合成4-(1H)-氰基咪唑核心的研究进展
我们已经开发了方便的两步一锅法轻松合成氰基咪唑的方法。通过集成一套反应监控技术,我们能够独立询问每个转换。我们观察到关键的半胱氨酸中间体的形成通过许多平衡过程,产生低聚物,最终导致无用的二聚化。DoE实验表明,尽管该底物具有复杂的平衡性,但仍可以实现高转化率的三氟甲基咪唑。我们还报告了温度,pH和底物浓度对消除级联反应(将三氟甲基咪唑转化为氰咪唑)的速率和选择性的影响。通过独立利用从每个步骤收集的动力学信息,我们报告了在一个锅中直接从含羰基的底物中获得高产率的氰基咪唑的反应条件。
更新日期:2020-07-28
中文翻译:

正交反应监测加速合成4-(1H)-氰基咪唑核心的研究进展
我们已经开发了方便的两步一锅法轻松合成氰基咪唑的方法。通过集成一套反应监控技术,我们能够独立询问每个转换。我们观察到关键的半胱氨酸中间体的形成通过许多平衡过程,产生低聚物,最终导致无用的二聚化。DoE实验表明,尽管该底物具有复杂的平衡性,但仍可以实现高转化率的三氟甲基咪唑。我们还报告了温度,pH和底物浓度对消除级联反应(将三氟甲基咪唑转化为氰咪唑)的速率和选择性的影响。通过独立利用从每个步骤收集的动力学信息,我们报告了在一个锅中直接从含羰基的底物中获得高产率的氰基咪唑的反应条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号