Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative analysis of yeast MAPK signaling networks and crosstalk using a microfluidic device.
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0lc00203h Byungjin Lee 1 , Seong-Geun Jeong , Si Hyung Jin , Ranjan Mishra , Matthias Peter , Chang-Soo Lee , Sung Sik Lee
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0lc00203h Byungjin Lee 1 , Seong-Geun Jeong , Si Hyung Jin , Ranjan Mishra , Matthias Peter , Chang-Soo Lee , Sung Sik Lee
Affiliation
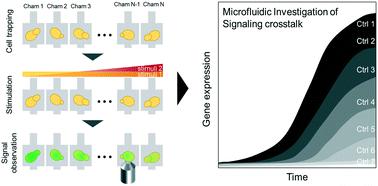
|
Eukaryotic cells developed complex mitogen-activated protein kinase (MAPK) signaling networks to sense their intra- and extracellular environment and respond to various stress conditions. For example, S. cerevisiae uses five distinct MAP kinase pathways to orchestrate meiosis or respond to mating pheromones, osmolarity changes and cell wall stress. Although each MAPK module has been studied individually, the mechanisms underlying crosstalk between signaling pathways remain poorly understood, in part because suitable experimental systems to monitor cellular outputs when applying different signals are lacking. Here, we investigate the yeast MAPK signaling pathways and their crosstalk, taking advantage of a new microfluidic device coupled to quantitative microscopy. We designed specific micropads to trap yeast cells in a single focal plane, and modulate the magnitude of a given stress signal by microfluidic serial dilution while keeping other signaling inputs constant. This approach enabled us to quantify in single cells nuclear relocation of effectors responding to MAPK activation, like Yap1 for oxidative stress, and expression of stress-specific reporter expression, like pSTL1-qV and pFIG1-qV for high-osmolarity or mating pheromone signaling, respectively. Using this quantitative single-cell analysis, we confirmed bimodal behavior of gene expression in response to Hog1 activation, and quantified crosstalk between the pheromone- and cell wall integrity (CWI) signaling pathways. Importantly, we further observed that oxidative stress inhibits pheromone signaling. Mechanistically, this crosstalk is mediated by Pkc1-dependent phosphorylation of the scaffold protein Ste5 on serine 185, which prevents Ste5 recruitment to the plasma membrane.
中文翻译:

使用微流控设备对酵母MAPK信号网络和串扰进行定量分析。
真核细胞开发了复杂的促分裂原活化蛋白激酶(MAPK)信号网络,以感知其细胞内和细胞外环境并应对各种应激条件。例如酿酒酵母使用五种不同的MAP激酶途径来协调减数分裂或响应交配的信息素,渗透压变化和细胞壁压力。尽管已经分别研究了每个MAPK模块,但对信号通路之间的串扰的潜在机制仍知之甚少,部分原因是缺少在施加不同信号时监测细胞输出的合适实验系统。在这里,我们利用结合定量显微镜的新型微流控设备,研究了酵母MAPK信号通路及其串扰。我们设计了特定的微孔板,以将酵母细胞捕获在单个焦平面中,并通过微流控系列稀释调节给定应力信号的强度,同时保持其他信号输入恒定。STL1 - qV和p FIG1 - qV分别用于高渗透压或交配信息素信号传递。使用这种定量的单细胞分析,我们确认了响应Hog1激活的基因表达的双峰行为,并量化了信息素和细胞壁完整性(CWI)信号通路之间的串扰。重要的是,我们进一步观察到氧化应激会抑制信息素的信号传导。从机制上讲,这种串扰是由丝氨酸185上支架蛋白Ste5的Pkc1依赖性磷酸化介导的,这阻止了Ste5募集到质膜上。
更新日期:2020-07-29
中文翻译:

使用微流控设备对酵母MAPK信号网络和串扰进行定量分析。
真核细胞开发了复杂的促分裂原活化蛋白激酶(MAPK)信号网络,以感知其细胞内和细胞外环境并应对各种应激条件。例如酿酒酵母使用五种不同的MAP激酶途径来协调减数分裂或响应交配的信息素,渗透压变化和细胞壁压力。尽管已经分别研究了每个MAPK模块,但对信号通路之间的串扰的潜在机制仍知之甚少,部分原因是缺少在施加不同信号时监测细胞输出的合适实验系统。在这里,我们利用结合定量显微镜的新型微流控设备,研究了酵母MAPK信号通路及其串扰。我们设计了特定的微孔板,以将酵母细胞捕获在单个焦平面中,并通过微流控系列稀释调节给定应力信号的强度,同时保持其他信号输入恒定。STL1 - qV和p FIG1 - qV分别用于高渗透压或交配信息素信号传递。使用这种定量的单细胞分析,我们确认了响应Hog1激活的基因表达的双峰行为,并量化了信息素和细胞壁完整性(CWI)信号通路之间的串扰。重要的是,我们进一步观察到氧化应激会抑制信息素的信号传导。从机制上讲,这种串扰是由丝氨酸185上支架蛋白Ste5的Pkc1依赖性磷酸化介导的,这阻止了Ste5募集到质膜上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号