当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Utilizing hydrogen underpotential deposition in CO reduction for highly selective formaldehyde production under ambient conditions
Green Chemistry ( IF 9.3 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0gc01412e Libo Yao 1, 2, 3, 4 , Yanbo Pan 1, 2, 3, 4 , Xiaochen Shen 1, 2, 3, 4 , Dezhen Wu 1, 2, 3, 4 , Abdulaziz Bentalib 1, 2, 3, 4 , Zhenmeng Peng 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0gc01412e Libo Yao 1, 2, 3, 4 , Yanbo Pan 1, 2, 3, 4 , Xiaochen Shen 1, 2, 3, 4 , Dezhen Wu 1, 2, 3, 4 , Abdulaziz Bentalib 1, 2, 3, 4 , Zhenmeng Peng 1, 2, 3, 4
Affiliation
|
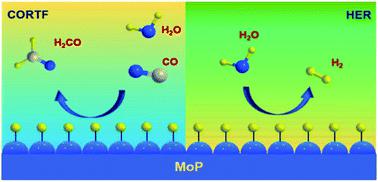
Formaldehyde is an essential building block for hundreds of chemicals and a promising liquid organic hydrogen carrier (LOHC), yet its indirect energy-intensive synthesis process prohibits it from playing a more significant role. Here we report a direct CO reduction to formaldehyde (CORTF) process that utilizes hydrogen underpotential deposition to overcome the thermodynamic barrier and the scaling relationship restriction. This is the first time that this reaction has been realized under ambient conditions. Using molybdenum phosphide as a catalyst, formaldehyde was produced with nearly a 100% faradaic efficiency in aqueous KOH solution, with its formation rate being one order of magnitude higher compared with the state-of-the-art thermal catalysis approach. Simultaneous tuning of the current density and reaction temperature led to a more selective and productive formaldehyde synthesis, indicating the electrochemical and thermal duality of this reaction. DFT calculations revealed that the desorption of the *H2CO intermediate likely served as the rate-limiting step, and the participation of H2O made the reaction thermodynamically favorable. Furthermore, a full-cell reaction set-up was demonstrated with CO hydrogenation to HCHO achieved without any energy input, which fully realized the spontaneous potential of the reaction. Our study shows the feasibility of combining thermal and electrochemical approaches for realizing the thermodynamics and for scaling relationship-confined reactions, which could serve as a new strategy in future reaction design.
中文翻译:

利用氢不足位沉积技术在CO还原过程中在环境条件下生产高选择性甲醛
甲醛是数百种化学药品的重要组成部分,也是一种有前途的液态有机氢载体(LOHC),但是其间接的能源密集型合成工艺使其无法发挥更大的作用。在这里,我们报告了直接的CO还原成甲醛(CORTF)的过程,该过程利用氢的欠电位沉积来克服热力学障碍和比例关系的限制。这是该反应在环境条件下首次实现。使用磷化钼作为催化剂,甲醛在KOH水溶液中的生成法拉第效率接近100%,与最新的热催化方法相比,甲醛的形成速率高一个数量级。同时调节电流密度和反应温度导致甲醛选择性和生产性更高,表明该反应的电化学和热对偶性。DFT计算表明* H的解吸2 CO中间体很可能是限速步骤,H 2 O的参与使反应在热力学上是有利的。此外,在没有任何能量输入的情况下,通过一氧化碳加氢生成HCHO的全电池反应装置得到了证实,这充分实现了反应的自发性。我们的研究表明,将热学和电化学方法相结合以实现热力学和缩放关系受限反应的可行性,这可以作为未来反应设计中的新策略。
更新日期:2020-08-31
中文翻译:

利用氢不足位沉积技术在CO还原过程中在环境条件下生产高选择性甲醛
甲醛是数百种化学药品的重要组成部分,也是一种有前途的液态有机氢载体(LOHC),但是其间接的能源密集型合成工艺使其无法发挥更大的作用。在这里,我们报告了直接的CO还原成甲醛(CORTF)的过程,该过程利用氢的欠电位沉积来克服热力学障碍和比例关系的限制。这是该反应在环境条件下首次实现。使用磷化钼作为催化剂,甲醛在KOH水溶液中的生成法拉第效率接近100%,与最新的热催化方法相比,甲醛的形成速率高一个数量级。同时调节电流密度和反应温度导致甲醛选择性和生产性更高,表明该反应的电化学和热对偶性。DFT计算表明* H的解吸2 CO中间体很可能是限速步骤,H 2 O的参与使反应在热力学上是有利的。此外,在没有任何能量输入的情况下,通过一氧化碳加氢生成HCHO的全电池反应装置得到了证实,这充分实现了反应的自发性。我们的研究表明,将热学和电化学方法相结合以实现热力学和缩放关系受限反应的可行性,这可以作为未来反应设计中的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号