当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity descriptors in acid catalysis: acid strength, proton affinity and host-guest interactions.
Chemical Communications ( IF 4.3 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0cc02593c Prashant Deshlahra 1 , Enrique Iglesia
Chemical Communications ( IF 4.3 ) Pub Date : 2020-06-22 , DOI: 10.1039/d0cc02593c Prashant Deshlahra 1 , Enrique Iglesia
Affiliation
|
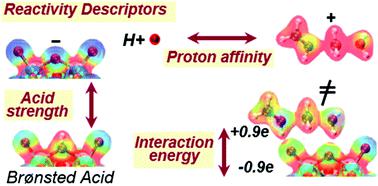
Brønsted acids mediate chemical transformations via proton transfer to bound species and interactions between the conjugate anion and bound cationic intermediates and transition states that are also stabilized by van der Waals forces within voids of molecular dimensions in inorganic hosts. This Feature Article describes the relevant descriptors of reactivity in terms of the properties of acids and molecules that determine their ability to donate and accept protons and to reorganize their respective charges to optimize their interactions at bound states. The deprotonation energy (DPE) of the acids and the protonation energy (Eprot) of the gaseous analogs of bound intermediates and transition states reflect their respective properties as species present at non-interacting distances. These properties accurately describe the reactivity of acids of a given type, such as polyoxometalates (POM) with a given type of addenda atom but different central atoms and heterosilicates, for different families of reactions. They do not fully capture, however, differences among acid types (e.g., Mo and W POM, heterosilicates, mineral acids) for diverse types of chemical transformations (e.g., elimination, isomerization, dimerization, condensation). The incompleteness of such descriptors reflects their inability to describe how protonated molecular species and conjugate anions restructure their respective charges when present as a binding pair at interacting distances. Such interaction energies represent electrostatic forces that depend on charge distributions in the cations and anions and the ability to reorganize the distributions to maximize the interactions. In the case of deprotonation, the electrostatic and charge reorganization components of DPE for various acids solely reflect the ability of the conjugate anion to accept and distribute the negative charge, a characteristic unique of each type of solid acid and specifically of the composition of its extended conjugate anion framework. The energy required to accept and rearrange the positive charge in bound intermediates and transition states reflects, in turn, their respective ability to recover the ionic and covalent components of DPE, the energy required to detach proton from conjugate anions. The DPE components and the recovery fractions together lead to a modified DPE, which captures only the part of DPE that remains unrecovered by the ion-pair interactions at bound intermediates and transition states, as the unifying descriptor for broad families of acids and reactions. The electrostatic and charge reorganization energies involved in these general descriptors are placed in historical context by assessing their connections to the heuristics of hard–soft acid–base displacements. Further development of these concepts requires benchmarking and extension of electrostatic and reorganization components of energies for a more diverse set of reaction types and acid families and advancement of methods for more efficient calculations of electrostatic interactions. Reactivity descriptors must also account for dispersive interactions between host cavities and guest molecules, requiring a framework analogous to the one described here for ion-pair interactions; these dispersive interactions depend on the fit between their shapes and sizes as well as their “structural stiffness” that determines the ability to modify the shapes of molecules and voids to minimize free energy. Entropy considerations and estimates of their dependence on properties of catalysts and molecules are also required for accurately determining Gibbs free energies that ultimately determine reaction rates.
中文翻译:

酸催化中的反应性描述符:酸强度,质子亲和力和主体与客体的相互作用。
布朗斯台德酸通过质子转移到结合的物种以及共轭阴离子与结合的阳离子中间体和过渡态之间的相互作用来介导化学转化,过渡态也通过范德华力在无机主体的分子尺寸空隙内稳定。这篇专题文章根据酸和分子的性质描述了反应性的相关描述符,这些酸和分子的性质决定了它们提供和接受质子以及重组其各自电荷以优化其在结合态的相互作用的能力。酸的去质子能(DPE)和质子能(E prot)的中间体和过渡态的气态类似物反映了它们各自的特性,即物种以非相互作用距离存在。这些性质准确地描述了给定类型的酸(例如具有给定类型的附加原子但具有不同中心原子和杂硅酸盐的多金属氧酸盐(POM))对于不同反应族的反应性。他们不完全捕捉,但是,酸类型(之间的差异例如,Mo和W POM,heterosilicates,无机酸)为不同类型的化学转化(如,消除,异构化,二聚,缩合)。这些描述符的不完整反映了它们无法描述质子化的分子种类和共轭阴离子如何以结合对的形式存在于相互作用距离时如何重组其各自的电荷。这样的相互作用能代表静电力,该静电力取决于阳离子和阴离子中的电荷分布以及重组分布以最大化相互作用的能力。在去质子化的情况下,DPE对各种酸的静电和电荷重组成分仅反映了共轭阴离子接受和分布负电荷的能力,这是每种类型的固体酸(尤其是其扩展的组成)所独有的特性共轭阴离子框架。在键合的中间体和过渡态中接受和重新排列正电荷所需的能量反过来又反映了它们各自回收DPE的离子和共价成分的能力,即将质子与共轭阴离子分离所需的能量。DPE组分和回收率馏分共同导致了改性DPE,它仅捕获了DPE的一部分,这些部分在结合的中间体和过渡态处因离子对相互作用而无法回收,这是广泛的酸和反应族的统一描述。这些一般描述符中涉及的静电和电荷重组能量通过评估它们与硬-软酸-基位移试探法的联系而置于历史背景中。这些概念的进一步发展要求对更多类型的反应类型和酸族进行标定和扩展能量的静电和重组成分,并需要改进方法以更有效地计算静电相互作用。反应性描述子还必须考虑到宿主腔和客体分子之间的分散相互作用,需要一种类似于此处所述的离子对相互作用的框架。这些分散的相互作用取决于它们的形状和大小之间的契合度,以及它们的“结构刚度”,后者决定了改变分子和空隙形状以最小化自由能的能力。
更新日期:2020-07-07
中文翻译:

酸催化中的反应性描述符:酸强度,质子亲和力和主体与客体的相互作用。
布朗斯台德酸通过质子转移到结合的物种以及共轭阴离子与结合的阳离子中间体和过渡态之间的相互作用来介导化学转化,过渡态也通过范德华力在无机主体的分子尺寸空隙内稳定。这篇专题文章根据酸和分子的性质描述了反应性的相关描述符,这些酸和分子的性质决定了它们提供和接受质子以及重组其各自电荷以优化其在结合态的相互作用的能力。酸的去质子能(DPE)和质子能(E prot)的中间体和过渡态的气态类似物反映了它们各自的特性,即物种以非相互作用距离存在。这些性质准确地描述了给定类型的酸(例如具有给定类型的附加原子但具有不同中心原子和杂硅酸盐的多金属氧酸盐(POM))对于不同反应族的反应性。他们不完全捕捉,但是,酸类型(之间的差异例如,Mo和W POM,heterosilicates,无机酸)为不同类型的化学转化(如,消除,异构化,二聚,缩合)。这些描述符的不完整反映了它们无法描述质子化的分子种类和共轭阴离子如何以结合对的形式存在于相互作用距离时如何重组其各自的电荷。这样的相互作用能代表静电力,该静电力取决于阳离子和阴离子中的电荷分布以及重组分布以最大化相互作用的能力。在去质子化的情况下,DPE对各种酸的静电和电荷重组成分仅反映了共轭阴离子接受和分布负电荷的能力,这是每种类型的固体酸(尤其是其扩展的组成)所独有的特性共轭阴离子框架。在键合的中间体和过渡态中接受和重新排列正电荷所需的能量反过来又反映了它们各自回收DPE的离子和共价成分的能力,即将质子与共轭阴离子分离所需的能量。DPE组分和回收率馏分共同导致了改性DPE,它仅捕获了DPE的一部分,这些部分在结合的中间体和过渡态处因离子对相互作用而无法回收,这是广泛的酸和反应族的统一描述。这些一般描述符中涉及的静电和电荷重组能量通过评估它们与硬-软酸-基位移试探法的联系而置于历史背景中。这些概念的进一步发展要求对更多类型的反应类型和酸族进行标定和扩展能量的静电和重组成分,并需要改进方法以更有效地计算静电相互作用。反应性描述子还必须考虑到宿主腔和客体分子之间的分散相互作用,需要一种类似于此处所述的离子对相互作用的框架。这些分散的相互作用取决于它们的形状和大小之间的契合度,以及它们的“结构刚度”,后者决定了改变分子和空隙形状以最小化自由能的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号