当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
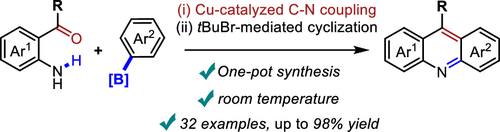
tert-Butyl Bromide-Promoted Intramolecular Cyclization of 2-Arylamino Phenyl Ketones and Its Combination with Cu-Catalyzed C-N Coupling: Synthesis of Acridines at Room Temperature.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.joc.0c00137 Zifeng Cao 1 , Yuan Zhu 1 , Xiaoman Li 1 , Yang He 1 , Jinli Zhang 1 , Liang Xu 1 , Yu Wei 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.joc.0c00137 Zifeng Cao 1 , Yuan Zhu 1 , Xiaoman Li 1 , Yang He 1 , Jinli Zhang 1 , Liang Xu 1 , Yu Wei 1
Affiliation
|
Herein, a facile intramolecular cyclization of 2-arylamino phenyl ketones is established to supersede the traditional high-temperature, strongly acidic conditions and achieve 9-substituted acridines, by virtue of the combination of 2,2,2-trifluoroethanol and tert-butyl bromide. This protocol can be merged well with the preceding Cu-catalyzed intermolecular Chan–Evans–Lam cross-coupling reactions, therefore enabling pot-economic modular synthesis of 9-substituted acridines from readily available 2-amino phenyl ketones and aryl boronic acids at room temperature.
中文翻译:

叔丁基溴化物促进的2-芳基氨基苯基酮的分子内环化及其与Cu催化的CN偶联的结合:室温下Synthesis啶的合成。
在此,借助于2,2,2-三氟乙醇和叔丁基溴的组合,建立了2-芳基氨基苯基酮的分子内环化体系,以取代传统的高温,强酸性条件并获得9-取代的a啶。。该方案可以与之前的铜催化的分子间Chan–Evans–Lam交叉偶联反应很好地合并,因此可以在室温下从容易获得的2-氨基苯基酮和芳基硼酸进行锅内经济的模块化合成9-取代的cr啶。
更新日期:2020-08-08
中文翻译:

叔丁基溴化物促进的2-芳基氨基苯基酮的分子内环化及其与Cu催化的CN偶联的结合:室温下Synthesis啶的合成。
在此,借助于2,2,2-三氟乙醇和叔丁基溴的组合,建立了2-芳基氨基苯基酮的分子内环化体系,以取代传统的高温,强酸性条件并获得9-取代的a啶。。该方案可以与之前的铜催化的分子间Chan–Evans–Lam交叉偶联反应很好地合并,因此可以在室温下从容易获得的2-氨基苯基酮和芳基硼酸进行锅内经济的模块化合成9-取代的cr啶。



























 京公网安备 11010802027423号
京公网安备 11010802027423号