当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Modular Chemoenzymatic Synthesis of Disialosyl Globopentaosylceramide (DSGb5Cer) Glycan.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.joc.0c01091 Dung-Yeh Wu,Avijit K Adak,Yan-Ting Kuo,Yu-Ju Shen,Pei-Jhen Li,Jih Ru Hwu,Chun-Cheng Lin
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.joc.0c01091 Dung-Yeh Wu,Avijit K Adak,Yan-Ting Kuo,Yu-Ju Shen,Pei-Jhen Li,Jih Ru Hwu,Chun-Cheng Lin
|
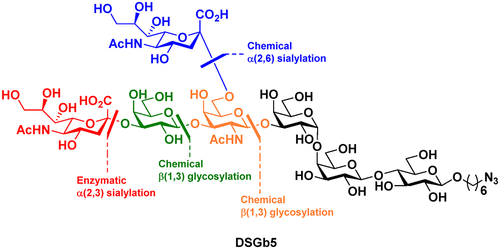
The total synthesis of the oligosaccharide moiety of disialosyl globopentaosylceramide (DSGb5 Cer), a dominant ganglioside isolated from malignant renal cell carcinoma tissues, is reported. The synthetic strategy relies on a chemical α(2,6)-sialylation at the internal GalNAc unit of a Gb5 pentasaccharide backbone that furnishes a Neu5Acα(2,6)GalNAc-linked hexasaccharide, suitable for an enzymatic α(2,3)-sialylation of the terminal Gal residue to construct a heptasaccharide glycan. Convergent access to this key α(2,6)-sialylated hexasaccharide was also achieved through a [3+3] glycosylation building upon a Galβ(1,3)[Neu5Acα(2,6)]GalNAc-based trisaccharide donor and a Gb3 acceptor. The synthetic DSGb5 glycan bearing a 6-azidohexyl aglycon at the reducing end could undergo further regioselective functionalization. This approach represents a viable chemoenzymatic method for accessing complex ganglioside glycans and should be useful for the synthesis and biological investigation of DSGb5 derivatives.
中文翻译:

二唾液酸 Globopentaosylceramide (DSGb5Cer) 聚糖的模块化化学酶法合成。
报道了从恶性肾细胞癌组织中分离出的主要神经节苷脂二唾液酸 globopentaosylceramide (DSGb5 Cer) 的寡糖部分的全合成。合成策略依赖于 Gb5 五糖骨架内部 GalNAc 单元的化学 α(2,6)-唾液酸化,提供 Neu5Acα(2,6)GalNAc 连接的六糖,适用于酶促 α(2,3)-末端 Gal 残基的唾液酸化以构建七糖聚糖。通过基于 Galβ(1,3)[Neu5Acα(2,6)]GalNAc 三糖供体和 Gb3 的 [3+3] 糖基化,也实现了对这一关键 α(2,6)-唾液酸化六糖的收敛访问受体。在还原端带有 6-叠氮己基苷元的合成 DSGb5 聚糖可以进行进一步的区域选择性功能化。
更新日期:2020-06-22
中文翻译:

二唾液酸 Globopentaosylceramide (DSGb5Cer) 聚糖的模块化化学酶法合成。
报道了从恶性肾细胞癌组织中分离出的主要神经节苷脂二唾液酸 globopentaosylceramide (DSGb5 Cer) 的寡糖部分的全合成。合成策略依赖于 Gb5 五糖骨架内部 GalNAc 单元的化学 α(2,6)-唾液酸化,提供 Neu5Acα(2,6)GalNAc 连接的六糖,适用于酶促 α(2,3)-末端 Gal 残基的唾液酸化以构建七糖聚糖。通过基于 Galβ(1,3)[Neu5Acα(2,6)]GalNAc 三糖供体和 Gb3 的 [3+3] 糖基化,也实现了对这一关键 α(2,6)-唾液酸化六糖的收敛访问受体。在还原端带有 6-叠氮己基苷元的合成 DSGb5 聚糖可以进行进一步的区域选择性功能化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号