当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of Acid–Base and Complexing Parameters of Chlorine-Substituted Trifluorobenzoylacetone in Water Medium
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.jced.0c00304 Maxim A. Lutoshkin 1, 2 , Yuriy N. Malyar 2, 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.jced.0c00304 Maxim A. Lutoshkin 1, 2 , Yuriy N. Malyar 2, 3
Affiliation
|
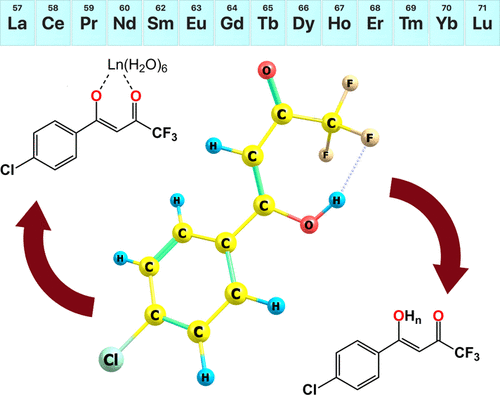
In this work, we study the behavior of 1-(4-chlorophenyl)-4,4,4-trifluoro-1,3-butanedione (ClTFBA) using a spectrophotometric technique under various conditions. Acid–base characteristics have been studied in citrate-phosphate buffer and hydrochloric acid solutions. Dissociation constants were determined in the pH range 3.0–8.0 for three ionic strengths (I = 0.1, 0.5, and 1.0 M; NaCl). Protonation equilibria have been investigated in concentrated HCl by the nonlinear Cox–Yeats method. The interaction between ClTFBA and rare-earth metals was examined in glycine and acetate buffers. Stability constants of monocomplexes were obtained for trivalent metals—Sc, Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu. The received formation constants lie from 4.5 to 11.8 logarithmic units. Analysis of the results shows that the thermodynamic stability of complexes increased from Ce(III) to Lu(III) and has a linear correlation with the ionic potential. The relationship described in this work demonstrates the electrostatic nature of the M(III)–ClTFBA bonds. Pronounced ionic character, observed for complexes with Ce, Pr, Nd, Sm, Eu, and Gd, points to high charge carrier mobility for these compounds and iterates the appropriateness to use the studied complexes to generate new organic light-emitting diode materials. Equilibrium and spectral characteristics were reproduced by density functional theory (DFT) and time-dependent DFT calculations performed using different basis sets and exchange–correlation functionals.
中文翻译:

水介质中氯取代的三氟苯甲酰丙酮的酸碱及络合参数的测定
在这项工作中,我们使用分光光度法研究了在各种条件下1-(4-氯苯基)-4,4,4-三氟-1,3-丁二酮(ClTFBA)的行为。在柠檬酸盐-磷酸盐缓冲液和盐酸溶液中已经研究了酸碱特性。在三种离子强度下,在3.0至8.0的pH范围内测定了解离常数(I= 0.1、0.5和1.0 M;NaCl)。已经通过非线性Cox–Yeats方法研究了浓HCl中的质子平衡。在甘氨酸和乙酸盐缓冲液中检查了ClTFBA和稀土金属之间的相互作用。获得了三价金属(Sc,Y,La,Ce,Pr,Nd,Sm,Eu,Gd,Tb,Dy,Ho,Er,Tm,Yb和Lu的单络合物的稳定常数。接收的地层常数为4.5到11.8对数单位。结果分析表明,配合物的热力学稳定性从Ce(III)增加到Lu(III),并与离子电势线性相关。这项工作中描述的关系证明了M(III)-ClTFBA键的静电性质。在与Ce,Pr,Nd,Sm,Eu和Gd形成配合物时观察到的明显的离子特征,这些化合物指出了这些化合物具有高的载流子迁移率,并重复了使用所研究的配合物生成新的有机发光二极管材料的适当性。平衡和光谱特性通过密度泛函理论(DFT)进行了复制,并使用不同的基集和交换相关函数进行了随时间变化的DFT计算。
更新日期:2020-07-09
中文翻译:

水介质中氯取代的三氟苯甲酰丙酮的酸碱及络合参数的测定
在这项工作中,我们使用分光光度法研究了在各种条件下1-(4-氯苯基)-4,4,4-三氟-1,3-丁二酮(ClTFBA)的行为。在柠檬酸盐-磷酸盐缓冲液和盐酸溶液中已经研究了酸碱特性。在三种离子强度下,在3.0至8.0的pH范围内测定了解离常数(I= 0.1、0.5和1.0 M;NaCl)。已经通过非线性Cox–Yeats方法研究了浓HCl中的质子平衡。在甘氨酸和乙酸盐缓冲液中检查了ClTFBA和稀土金属之间的相互作用。获得了三价金属(Sc,Y,La,Ce,Pr,Nd,Sm,Eu,Gd,Tb,Dy,Ho,Er,Tm,Yb和Lu的单络合物的稳定常数。接收的地层常数为4.5到11.8对数单位。结果分析表明,配合物的热力学稳定性从Ce(III)增加到Lu(III),并与离子电势线性相关。这项工作中描述的关系证明了M(III)-ClTFBA键的静电性质。在与Ce,Pr,Nd,Sm,Eu和Gd形成配合物时观察到的明显的离子特征,这些化合物指出了这些化合物具有高的载流子迁移率,并重复了使用所研究的配合物生成新的有机发光二极管材料的适当性。平衡和光谱特性通过密度泛函理论(DFT)进行了复制,并使用不同的基集和交换相关函数进行了随时间变化的DFT计算。











































 京公网安备 11010802027423号
京公网安备 11010802027423号