当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-perfectly Amphipathic α-Helical Structure Containing the XXYXX Sequence Improves the Biological Activity of Bovine αs2-Casein Antimicrobial Peptides.
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.jafc.0c01377 Liya Gu 1 , Changbao Sun 1 , Lijun Chen 2 , Shiyue Pang 1 , Muhammad Altaf Hussain 1 , Chenggang Jiang 3 , Jiage Ma 1 , Zhanmei Jiang 1 , Juncai Hou 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.jafc.0c01377 Liya Gu 1 , Changbao Sun 1 , Lijun Chen 2 , Shiyue Pang 1 , Muhammad Altaf Hussain 1 , Chenggang Jiang 3 , Jiage Ma 1 , Zhanmei Jiang 1 , Juncai Hou 1
Affiliation
|
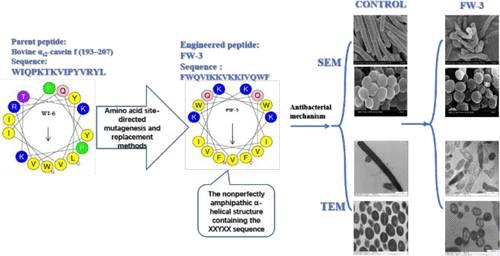
Non-amphiphilic WIQPKTKVIPYVRYL (WI-6) derived from bovine αs2-casein f (193–207) was modified by a defined mutation method to obtain five engineered peptides with mirror symmetry structures. The five engineered peptide sequences were WF-1 (WFQVKTRVRTKVQFW), FW-2 (FWRRYKKVKKYRRWF), FW-3 (FWQVIKKVKKIVQWF), FK-4 (FKQFYRRVRRYFQKF), and FR-5 (FRQWYRRVRRYWQRF). However, FW-2, FW-3, FK-4, and FR-5 had obvious XXYXX sequences. Among these, FW-3 was demonstrated to have the highest antibacterial activity, which indicates that the non-perfectly amphipathic α-helical structure containing the XXYXX sequence has a better bactericidal effect. Therefore, peptide FW-3 could be widely used as a substitute for antibiotics in food, medicine, and other fields. These findings provide a potential method for designing novel antimicrobial peptides.
中文翻译:

包含XXYXX序列的非完美两亲性α-螺旋结构可改善牛αs2-酪蛋白抗菌肽的生物活性。
非两亲WIQPKTKVIPYVRYL(WI-6)从牛α衍生S2-酪蛋白f(193-207)通过定义的突变方法进行修饰,以获得五种具有镜面对称结构的工程化肽。这五个工程化的肽序列是WF-1(WFQVKTRVRTKTKVQFW),FW-2(FWRRYKKVKKYRRWF),FW-3(FWQVIKKVKKIVQWF),FK-4(FKQFYRRVRRYFQKF)和FR-5(FRQWYRRVRRYWQRF)。但是,FW-2,FW-3,FK-4和FR-5具有明显的XXYXX序列。其中,FW-3被证明具有最高的抗菌活性,这表明含有XXYXX序列的非完美两亲性α-螺旋结构具有更好的杀菌效果。因此,肽FW-3可以广泛用作食品,药品和其他领域中抗生素的替代品。这些发现为设计新型抗菌肽提供了一种潜在的方法。
更新日期:2020-07-15
中文翻译:

包含XXYXX序列的非完美两亲性α-螺旋结构可改善牛αs2-酪蛋白抗菌肽的生物活性。
非两亲WIQPKTKVIPYVRYL(WI-6)从牛α衍生S2-酪蛋白f(193-207)通过定义的突变方法进行修饰,以获得五种具有镜面对称结构的工程化肽。这五个工程化的肽序列是WF-1(WFQVKTRVRTKTKVQFW),FW-2(FWRRYKKVKKYRRWF),FW-3(FWQVIKKVKKIVQWF),FK-4(FKQFYRRVRRYFQKF)和FR-5(FRQWYRRVRRYWQRF)。但是,FW-2,FW-3,FK-4和FR-5具有明显的XXYXX序列。其中,FW-3被证明具有最高的抗菌活性,这表明含有XXYXX序列的非完美两亲性α-螺旋结构具有更好的杀菌效果。因此,肽FW-3可以广泛用作食品,药品和其他领域中抗生素的替代品。这些发现为设计新型抗菌肽提供了一种潜在的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号