当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytoskeletal Drugs Modulate Off-Target Protein Folding Landscapes Inside Cells.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.biochem.0c00299 Caitlin M Davis , Martin Gruebele
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.biochem.0c00299 Caitlin M Davis , Martin Gruebele
|
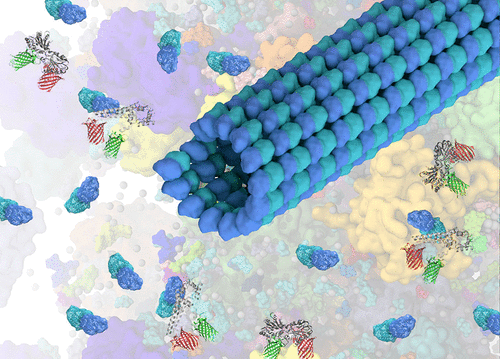
The dynamic cytoskeletal network of microtubules and actin filaments can be disassembled by drugs. Cytoskeletal drugs work by perturbing the monomer–polymer equilibrium, thus changing the size and number of macromolecular crowders inside cells. Changes in both crowding and nonspecific surface interactions (“sticking”) following cytoskeleton disassembly can affect the protein stability, structure, and function directly or indirectly by changing the fluidity of the cytoplasm and altering the crowding and sticking of other macromolecules in the cytoplasm. The effect of cytoskeleton disassembly on protein energy landscapes inside cells has yet to be observed. Here we have measured the effect of several cytoskeletal drugs on the folding energy landscape of two FRET-labeled proteins with different in vitro sensitivities to macromolecular crowding. Phosphoglycerate kinase (PGK) was previously shown to be more sensitive to crowding, whereas variable major protein-like sequence expressed (VlsE) was previously shown to be more sensitive to sticking. The in-cell effects of drugs that depolymerize either actin filaments (cytochalasin D and latrunculin B) or microtubules (nocodazole and vinblastine) were compared. The crowding sensor protein CrH2-FRET verified that cytoskeletal drugs decrease the extent of crowding inside cells despite also reducing the overall cell volume. The decreased compactness and folding stability of PGK could be explained by the decreased extent of crowding induced by these drugs. VlsE’s opposite response to the drugs shows that depolymerization of the cytoskeleton also changes sticking in the cellular milieu. Our results demonstrate that perturbation of the monomer–polymer cytoskeletal equilibrium, for example, during natural cell migration or stresses from drug treatment, has off-target effects on the energy landscapes of proteins in the cell.
中文翻译:

细胞骨架药物调节细胞内脱靶蛋白折叠态。
微管和肌动蛋白丝的动态细胞骨架网络可以通过药物分解。细胞骨架药物通过干扰单体-聚合物平衡来起作用,从而改变细胞内大分子拥挤物的大小和数量。通过改变细胞质的流动性并改变细胞质中其他大分子的拥挤和粘附,细胞骨架拆卸后拥挤和非特异性表面相互作用(“粘附”)的变化都可以直接或间接影响蛋白质的稳定性,结构和功能。细胞骨架分解对细胞内蛋白质能量分布的影响尚未观察到。在这里,我们测量了几种细胞骨架药物对两种FRET标记蛋白在体外具有不同折叠能量的影响对大分子拥挤的敏感性。先前显示磷酸甘油酸激酶(PGK)对拥挤更敏感,而先前表达的可变主要蛋白样序列(VlsE)对粘附更敏感。比较了使肌动蛋白丝(细胞松弛素D和latrunculin B)或微管(诺考达唑和长春碱)解聚的药物的细胞内作用。拥挤传感器蛋白CrH2-FRET证实,尽管也减少了总细胞体积,但细胞骨架药物减少了细胞内部的拥挤程度。PGK的致密性和折叠稳定性降低是由于这些药物引起的拥挤程度降低。VlsE对药物的相反反应表明,细胞骨架的解聚也改变了细胞环境中的黏附。
更新日期:2020-07-21
中文翻译:

细胞骨架药物调节细胞内脱靶蛋白折叠态。
微管和肌动蛋白丝的动态细胞骨架网络可以通过药物分解。细胞骨架药物通过干扰单体-聚合物平衡来起作用,从而改变细胞内大分子拥挤物的大小和数量。通过改变细胞质的流动性并改变细胞质中其他大分子的拥挤和粘附,细胞骨架拆卸后拥挤和非特异性表面相互作用(“粘附”)的变化都可以直接或间接影响蛋白质的稳定性,结构和功能。细胞骨架分解对细胞内蛋白质能量分布的影响尚未观察到。在这里,我们测量了几种细胞骨架药物对两种FRET标记蛋白在体外具有不同折叠能量的影响对大分子拥挤的敏感性。先前显示磷酸甘油酸激酶(PGK)对拥挤更敏感,而先前表达的可变主要蛋白样序列(VlsE)对粘附更敏感。比较了使肌动蛋白丝(细胞松弛素D和latrunculin B)或微管(诺考达唑和长春碱)解聚的药物的细胞内作用。拥挤传感器蛋白CrH2-FRET证实,尽管也减少了总细胞体积,但细胞骨架药物减少了细胞内部的拥挤程度。PGK的致密性和折叠稳定性降低是由于这些药物引起的拥挤程度降低。VlsE对药物的相反反应表明,细胞骨架的解聚也改变了细胞环境中的黏附。











































 京公网安备 11010802027423号
京公网安备 11010802027423号