当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nucleosome Binding by the Lysine Specific Demethylase 1 (LSD1) Enzyme Enables Histone H3 Demethylation.
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.biochem.0c00412 Abhinav Dhall 1 , Patrick M M Shelton 1 , Aurore M-F Delachat 2 , Calvin J A Leonen 1 , Beat Fierz 2 , Champak Chatterjee 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-06-22 , DOI: 10.1021/acs.biochem.0c00412 Abhinav Dhall 1 , Patrick M M Shelton 1 , Aurore M-F Delachat 2 , Calvin J A Leonen 1 , Beat Fierz 2 , Champak Chatterjee 1
Affiliation
|
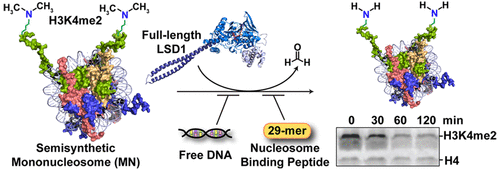
The essential human enzyme lysine specific demethylase 1 (LSD1) silences genes by demethylating mono- and dimethylated lysine 4 in histone H3 (H3K4me1/2). Studies of the minimal requirements for LSD1 activity are complicated by the heterogeneity of histone modification states in cells. We overcame this challenge by generating homogeneous mononucleosome substrates containing semisynthetic H3K4me2. Biophysical and biochemical assays with full-length LSD1 revealed its ability to bind and demethylate nucleosomes. Consistent with a requirement for nucleosome binding prior to demethylation, a competing nucleosome-binding peptide from the high-mobility group protein effectively inhibited LSD1 activity. Thus, our studies provide the first glimpse of nucleosome demethylation by LSD1 in the absence of other scaffolding proteins.
中文翻译:

赖氨酸特异性脱甲基酶1(LSD1)酶的核小体结合使组蛋白H3脱甲基。
人类必需的赖氨酸特异性脱甲基酶1(LSD1)通过使组蛋白H3(H3K4me1 / 2)中的单和二甲基赖氨酸4脱甲基来沉默基因。LSD1活性最低要求的研究因细胞中组蛋白修饰状态的异质性而变得复杂。我们通过产生包含半合成H3K4me2的均质单核小体底物克服了这一挑战。全长LSD1的生物物理和生化分析表明,它具有结合和脱甲基核小体的能力。与去甲基化之前对核小体结合的要求一致,来自高迁移率基团蛋白的竞争性核小体结合肽可有效抑制LSD1活性。因此,我们的研究提供了在没有其他支架蛋白的情况下,LSD1对核小体脱甲基作用的首次了解。
更新日期:2020-07-14
中文翻译:

赖氨酸特异性脱甲基酶1(LSD1)酶的核小体结合使组蛋白H3脱甲基。
人类必需的赖氨酸特异性脱甲基酶1(LSD1)通过使组蛋白H3(H3K4me1 / 2)中的单和二甲基赖氨酸4脱甲基来沉默基因。LSD1活性最低要求的研究因细胞中组蛋白修饰状态的异质性而变得复杂。我们通过产生包含半合成H3K4me2的均质单核小体底物克服了这一挑战。全长LSD1的生物物理和生化分析表明,它具有结合和脱甲基核小体的能力。与去甲基化之前对核小体结合的要求一致,来自高迁移率基团蛋白的竞争性核小体结合肽可有效抑制LSD1活性。因此,我们的研究提供了在没有其他支架蛋白的情况下,LSD1对核小体脱甲基作用的首次了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号